Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Real-Time In Situ Secondary Structure Analysis of Protein Monolayer with Mid-Infrared Plasmonic Nanoantennas.
ACS Sensors ( IF 8.2 ) Pub Date : 2018-06-12 , DOI: 10.1021/acssensors.8b00115 Dordaneh Etezadi , John B. Warner , Hilal A. Lashuel , Hatice Altug
ACS Sensors ( IF 8.2 ) Pub Date : 2018-06-12 , DOI: 10.1021/acssensors.8b00115 Dordaneh Etezadi , John B. Warner , Hilal A. Lashuel , Hatice Altug
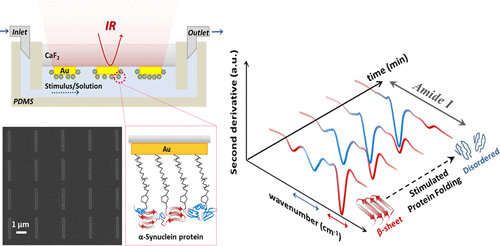
|
Dynamic detection of protein conformational changes at physiological conditions on a minute amount of samples is immensely important for understanding the structural determinants of protein function in health and disease and to develop assays and diagnostics for protein misfolding and protein aggregation diseases. Herein, we experimentally demonstrate the capabilities of a mid-infrared plasmonic biosensor for real-time and in situ protein secondary structure analysis in aqueous environment at nanoscale. We present label-free ultrasensitive dynamic monitoring of β-sheet to disordered conformational transitions in a monolayer of the disease-related α-synuclein protein under varying stimulus conditions. Our experiments show that the extracted secondary structure signals from plasmonically enhanced amide I signatures in the protein monolayer can be reliably and reproducibly acquired with second derivative analysis for dynamic monitoring. Furthermore, by using a polymer layer we show that our nanoplasmonic approach of extracting the frequency components of vibrational signatures matches with the results attained from gold-standard infrared transmission measurements. By facilitating conformational analysis on small quantities of immobilized proteins in response to external stimuli such as drugs, our plasmonic biosensor could be used to introduce platforms for screening small molecule modulators of protein misfolding and aggregation.
中文翻译:

中红外等离子纳米天线对蛋白质单层的实时原位二级结构分析。
在生理条件下对微量样品进行蛋白质构象变化的动态检测对于理解健康和疾病中蛋白质功能的结构决定因素以及开发针对蛋白质错误折叠和蛋白质聚集疾病的测定方法和诊断方法极为重要。在这里,我们通过实验证明了中红外等离子体生物传感器在纳米级水环境中实时和原位蛋白质二级结构分析的能力。我们目前在变化的刺激条件下,疾病相关的α-突触核蛋白单层中无序构象转变的无标签超灵敏动态监测β-片层。我们的实验表明,可以通过二阶导数分析进行动态监测,从蛋白质单分子层中从等离激元增强的酰胺I签名中提取的二级结构信号可以可靠且可重现。此外,通过使用聚合物层,我们证明了提取振动信号频率分量的纳米等离子体方法与从金标准红外透射测量获得的结果相匹配。通过促进对诸如药物等外部刺激的少量固定化蛋白质的构象分析,我们的等离激元生物传感器可用于引入筛选蛋白质错折叠和聚集的小分子调节剂的平台。通过使用聚合物层,我们证明了提取振动信号频率分量的纳米等离子体方法与金标准红外透射测量获得的结果相匹配。通过促进对诸如药物等外部刺激的少量固定化蛋白的构象分析,我们的等离激元生物传感器可用于引入筛选蛋白错折叠和聚集的小分子调节剂的平台。通过使用聚合物层,我们证明了提取振动信号频率分量的纳米等离子体方法与金标准红外透射测量获得的结果相匹配。通过促进对诸如药物等外部刺激的少量固定化蛋白的构象分析,我们的等离激元生物传感器可用于引入筛选蛋白错折叠和聚集的小分子调节剂的平台。
更新日期:2018-05-30
中文翻译:

中红外等离子纳米天线对蛋白质单层的实时原位二级结构分析。
在生理条件下对微量样品进行蛋白质构象变化的动态检测对于理解健康和疾病中蛋白质功能的结构决定因素以及开发针对蛋白质错误折叠和蛋白质聚集疾病的测定方法和诊断方法极为重要。在这里,我们通过实验证明了中红外等离子体生物传感器在纳米级水环境中实时和原位蛋白质二级结构分析的能力。我们目前在变化的刺激条件下,疾病相关的α-突触核蛋白单层中无序构象转变的无标签超灵敏动态监测β-片层。我们的实验表明,可以通过二阶导数分析进行动态监测,从蛋白质单分子层中从等离激元增强的酰胺I签名中提取的二级结构信号可以可靠且可重现。此外,通过使用聚合物层,我们证明了提取振动信号频率分量的纳米等离子体方法与从金标准红外透射测量获得的结果相匹配。通过促进对诸如药物等外部刺激的少量固定化蛋白质的构象分析,我们的等离激元生物传感器可用于引入筛选蛋白质错折叠和聚集的小分子调节剂的平台。通过使用聚合物层,我们证明了提取振动信号频率分量的纳米等离子体方法与金标准红外透射测量获得的结果相匹配。通过促进对诸如药物等外部刺激的少量固定化蛋白的构象分析,我们的等离激元生物传感器可用于引入筛选蛋白错折叠和聚集的小分子调节剂的平台。通过使用聚合物层,我们证明了提取振动信号频率分量的纳米等离子体方法与金标准红外透射测量获得的结果相匹配。通过促进对诸如药物等外部刺激的少量固定化蛋白的构象分析,我们的等离激元生物传感器可用于引入筛选蛋白错折叠和聚集的小分子调节剂的平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号