当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Temperature Rate Constants for the Reaction of Hydrogen Atoms with Tetramethoxysilane and Reactivity Analogies between Silanes and Oxygenated Hydrocarbons
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-30 00:00:00 , DOI: 10.1021/acs.jpca.8b03160 Sebastian Peukert 1 , Pavel Yatsenko 2 , Mustapha Fikri 1 , Christof Schulz 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-30 00:00:00 , DOI: 10.1021/acs.jpca.8b03160 Sebastian Peukert 1 , Pavel Yatsenko 2 , Mustapha Fikri 1 , Christof Schulz 1
Affiliation
|
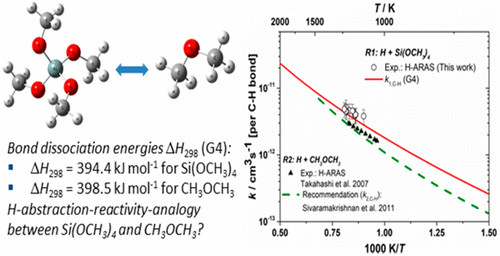
The shock-tube technique has been used to investigate the H-abstraction reaction H + Si(OCH3)4 → H2 + Si(OCH2)(OCH3)3 behind reflected shock waves. C2H5I was used as a thermal in situ source for H atoms. The experiments covered a temperature range of 1111–1238 K, and pressures of 1.3–1.4 bar. H atom concentrations were monitored with atomic resonance absorption spectrometry (ARAS). Fits to the temporal H atom concentration profiles based on a developed chemical kinetics reaction mechanism were used for determining bimolecular rate constants. Experimental total H-abstraction rate constants were well represented by the Arrhenius equation ktotal(T) = 10–9.16±0.24 exp(−25.5 ± 5.6 kJ mol–1/RT) cm3 s–1. Transition state theory (TST) calculations based on the G4 level of theory show excellent agreement with experimentally obtained rate constants, i.e., the theory values of k(T) deviate by less than 25% from the experimental results. Regarding H abstractions, we have compared the reactivity of C–H bonds in Si(OCH3)4 with the reactivity of C–H bonds in dimethyl ether (CH3OCH3). Present experimental and theoretical results indicate that at high temperatures, i.e., T > 500 K, CH3OCH3 is a good reactivity analog to Si(OCH3)4, i.e., kH+Si(OCH3)4(T) ∼ 1.5 × kH+CH3OCH3(T). On the basis of these results, we discuss the possibility of drawing reactivity analogies between oxygenated silanes and oxygenated hydrocarbons.
中文翻译:

氢原子与四甲氧基硅烷反应的高温速率常数以及硅烷与氧化烃之间的反应性类似物
冲击管技术已被用于研究反射冲击波背后的H吸收反应H + Si(OCH 3)4 →H 2 + Si(OCH 2)(OCH 3)3。C 2 H 5 I用作原位热H原子的来源。实验覆盖的温度范围为1111-1238 K,压力为1.3-1.4 bar。用原子共振吸收光谱法(ARAS)监测H原子浓度。根据发达的化学动力学反应机理,对时间氢原子浓度分布进行拟合,以确定双分子速率常数。实验的总H吸收速率常数由Arrhenius方程很好地表示出来k total(T)= 10 –9.16±0.24 exp(−25.5±5.6 kJ mol –1 / RT)cm 3 s –1。基于G4理论水平的过渡态理论(TST)计算显示出与实验获得的速率常数极佳的一致性,即k(T)的理论值与实验结果的偏差小于25%。关于H的抽象,我们比较了Si(OCH 3)4中C–H键的反应性与二甲醚(CH 3 OCH 3)中C–H键的反应性。当前的实验和理论结果表明,在高温下,即T > 500 K,CH 3 OCH 3是类似于Si(OCH 3)4的良好反应性,即kH + Si(OCH3)4(T)〜1.5 × k H + CH3OCH3(T)。基于这些结果,我们讨论了在氧化硅烷和氧化烃之间进行反应性类比的可能性。
更新日期:2018-05-30
中文翻译:

氢原子与四甲氧基硅烷反应的高温速率常数以及硅烷与氧化烃之间的反应性类似物
冲击管技术已被用于研究反射冲击波背后的H吸收反应H + Si(OCH 3)4 →H 2 + Si(OCH 2)(OCH 3)3。C 2 H 5 I用作原位热H原子的来源。实验覆盖的温度范围为1111-1238 K,压力为1.3-1.4 bar。用原子共振吸收光谱法(ARAS)监测H原子浓度。根据发达的化学动力学反应机理,对时间氢原子浓度分布进行拟合,以确定双分子速率常数。实验的总H吸收速率常数由Arrhenius方程很好地表示出来k total(T)= 10 –9.16±0.24 exp(−25.5±5.6 kJ mol –1 / RT)cm 3 s –1。基于G4理论水平的过渡态理论(TST)计算显示出与实验获得的速率常数极佳的一致性,即k(T)的理论值与实验结果的偏差小于25%。关于H的抽象,我们比较了Si(OCH 3)4中C–H键的反应性与二甲醚(CH 3 OCH 3)中C–H键的反应性。当前的实验和理论结果表明,在高温下,即T > 500 K,CH 3 OCH 3是类似于Si(OCH 3)4的良好反应性,即kH + Si(OCH3)4(T)〜1.5 × k H + CH3OCH3(T)。基于这些结果,我们讨论了在氧化硅烷和氧化烃之间进行反应性类比的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号