当前位置:
X-MOL 学术
›
Fuel Process. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantification of reactive intermediate radicals and their induction effect during pyrolysis of two n -alkylbenzenes
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.fuproc.2018.05.025 Zezhou Chen , Xurui Zhang , Zhenyu Liu , Qingya Liu , Teng Xu
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2018-09-01 , DOI: 10.1016/j.fuproc.2018.05.025 Zezhou Chen , Xurui Zhang , Zhenyu Liu , Qingya Liu , Teng Xu
|
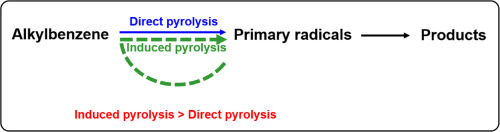
Abstract Pyrolysis of heavy organic resources follows the radical mechanism. It involves the reactions between radicals generated from the cleavage of covalent bonds in a reactant and the reactions between the radicals and the reactant. The radicals are the reactive intermediates that govern the rate and product distribution of pyrolysis. This paper quantifies the reactive intermediate radicals generated in direct pyrolysis of two n -alkylbenzenes and the induced pyrolysis of the alkylbenzenes by the radicals, based on the data obtained in pyrolysis with and without tetralin that is able to donate hydrogen radicals. It is found that about 90% reactive intermediate radicals can be capped by the hydrogen radicals from tetralin. At 400–440 °C the contribution of induced pyrolysis is more than that of direct pyrolysis, about 1.9 times for n -propylbenzene and 3 times for n -pentylbenzene. The role of induced pyrolysis is larger at higher temperatures due to its higher activation energy than direct pyrolysis.
更新日期:2018-09-01











































 京公网安备 11010802027423号
京公网安备 11010802027423号