Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-05-29 , DOI: 10.1016/j.mcat.2018.05.024 Alexandra M. Zima , Oleg Y. Lyakin , Konstantin P. Bryliakov , Evgenii P. Talsi
|
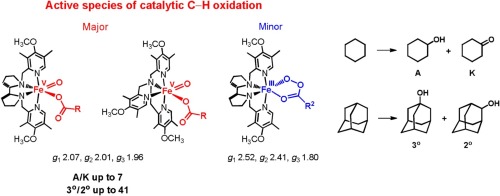
Iron complexes of the PDP family [((S,S)-PDP)FeII(OTf)2] (1) and [((S,S)-PDP*)FеIII(μ-OH)2FеIII((S,S)-PDР*)](ОTf)4 (3), (S,S)-PDР = N,N′-bis(2-pyridylmethyl)-(S,S)-2,2′-biрyrrоlidine, (S,S)-PDР* = N,N′-bis(3,5-dimеthyl-4-mеthоxypyridyl-2-mеthyl)-(S,S)-2,2′-biрyrrоlidine, and of the TPA family [(TPА)FeII(CH3СN)2](СlO4)2 (4) and [(TPА*)FеIII(μ-OH)2FеIII(TPА*)](ОTf)4 (5), TPА = tris(2-pyridylmethyl)amine, TPA* = tris(3,5-dimеthyl-4-mеthoxyрyridyl-2-methyl)аmine, catаlyze the selеctive hydrоxylation of alkаnes with hydrogen peroxide and peroxycarboxylic acids as terminal oxidants. The nature of the active species of these catalytic systems has been evaluated by combined EPR spectroscopic and catalytic studies. To this end, the catalytic systems Fe complex/oxidant/RCOOH (catalyst: 1, 3, 4, 5; oxidant: H2O2, CH3CO3H, m-chloroperoxybenzoic acid = m-CPBA; RCOOH: acetic acid = AA, 2-ethylhexanoic acid = EHA), exhibiting EPR spectra of iron-oxo and/or iron-acylperoxo intermediates, have been systematically studied in the chemoselective oxidation of cyclohexane and regioselective oxidation of adamantane. In the latter case, high yield of oxidation products (up to 67 TN per Fe atom, or 67%) and high regioselectivity (3°/2° up to 41) were observed. Depending on the nature of the catalyst, oxidant and catalytic additive, various iron-oxygen intermediates have been observed in the catalytic systems studied. Iron(V)-oxo intermediates have been suggested to be the major active species of C–H hydroxylation by the systems catalyst/H2O2/RCOOH and catalyst/CH3CO3H/RCOOH. In contrast to the catalytic systems with H2O2 and CH3CO3H as oxidants, in the systems relying on m-CPBA, the contribution of iron-acylperoxo intermediates into the oxidation may be significant.
中文翻译:

活性中间体在铁催化过氧化氢和过酸氧化环烷烃中的性质
该PDP家族的铁配合物[((小号,小号)-PDP)的Fe II(OTF)2 ](1)和[((小号,小号)-PDP *)Fе III(μ -OH)2 Fе III((S,S)-PDР*)](ОTf)4(3),(S,S)-PDР= N,N'-双(2-吡啶基甲基)-(S,S)-2,2'-联苯吡啶(S,S)-PDР* = N,N双(3,5-dimеthyl-4-mеthоxypyridyl-2-mеthyl) - (小号,小号)-2,2'-biрyrrоlidine,和TPA家族的[(TPА)的Fe II(CH 3 СN)2 ] (СlO 4)2(4)和[(TPА*)Fе III(μ-OH)2 Fе III(TPА*)](ОTf)4(5),TPА=三(2-吡啶基甲基)胺,TPA * =三(3,5-二甲基-4-间甲氧基-吡啶基-2-甲基)胺,用过氧化氢和过氧羧酸作为末端氧化剂催化烷基的选择性羟化反应。这些催化体系的活性物种的性质已经通过结合EPR光谱学和催化研究进行了评估。为此,在催化系统铁络合物/氧化剂/ RCOOH(催化剂:1,3,4,5 ;氧化剂:H 2 ö 2,CH 3 CO 3 H,中号氯过氧酸= 米-CPBA;RCOOH:乙酸= AA,2-乙基己酸= EHA),在环己烷的化学选择氧化和金刚烷的区域选择性氧化中,系统地研究了具有铁-氧和/或铁-酰基过氧中间体的EPR光谱。在后一种情况下,观察到氧化产物的产率高(每个Fe原子高达67 TN,或67%)和区域选择性高(3°/ 2°,高达41)。取决于催化剂,氧化剂和催化添加剂的性质,在所研究的催化体系中已观察到各种铁-氧中间体。通过催化剂/ H 2 O 2 / RCOOH和催化剂/ CH 3 CO 3的体系,已表明铁(V)-氧代中间体是C–H羟基化的主要活性物质。H / RCOOH。与使用H 2 O 2和CH 3 CO 3 H作为氧化剂的催化体系相反,在依赖m -CPBA的体系中,铁酰基过氧中间体对氧化的贡献可能很大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号