当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Constructing Unique Cathode Interface by Manipulating Functional Groups of Electrolyte Additive for Graphite/LiNi0.6Co0.2Mn0.2O2 Cells at High Voltage
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.jpclett.8b01099 Bo Liao 1 , Xinliang Hu 2 , Mengqing Xu 1 , Hongying Li 1 , Le Yu 3 , Weizhen Fan 3 , Lidan Xing 1 , Youhao Liao 1 , Weishan Li 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.jpclett.8b01099 Bo Liao 1 , Xinliang Hu 2 , Mengqing Xu 1 , Hongying Li 1 , Le Yu 3 , Weizhen Fan 3 , Lidan Xing 1 , Youhao Liao 1 , Weishan Li 1
Affiliation
|
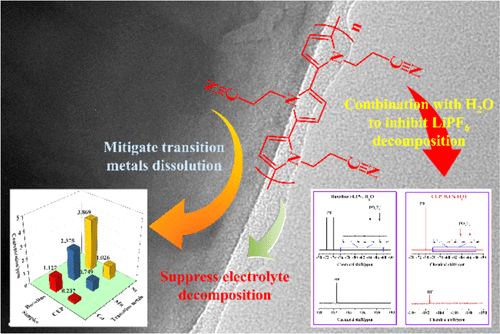
A novel electrolyte additive, 1-(2-cyanoethyl) pyrrole (CEP), has been investigated to improve the electrochemical performance of graphite/LiNi0.6Co0.2Mn0.2O2 cells cycling up to 4.5 V vs Li/Li+. The 4.5 V cycling results present that after 50 cycles, up to 4.5 V capacity retention of the graphite/LiNi0.6Co0.2Mn0.2O2 cell is improved significantly from 27.4 to 81.5% when adding 1% CEP to baseline electrolyte (1 M LiPF6 in EC/EMC 1:2, by weight). Ex situ characterization results support the mechanism of CEP for enhancing the electrochemical performance. On one hand, the significant enhancement is ascribed to a formed superior cathode interfacial film by preferential oxidation of CEP on the cathode electrode surface suppressing electrolyte decomposition at high voltage. On the other hand, the duo Lewis base functional groups can effectively capture dissociation product PF5 from LiPF6 with the presence of an unavoidable trace amount of water or aprotic impurities in the electrolyte. Thus this mitigates the hydrofluoric acid (HF) generation that leads to the reduction of transition-metal dissolution in the electrolyte upon cycling at high voltage. The theoretical modeling suggests that CEP has a mechanism of stabilizing electrolyte via combination of —C≡N: functional group and H2O. The work presented here also shows nuclear magnetic resonance spectra analysis to prove the capability of CEP reducing HF generation and X-ray photoelectron spectroscopy analysis to observe cathode surface composition.
中文翻译:

通过操纵高压下石墨/ LiNi 0.6 Co 0.2 Mn 0.2 O 2电池电解质添加剂的官能团来构建独特的阴极界面
已经研究了一种新型的电解质添加剂1-(2-氰乙基)吡咯(CEP),以改善相对于Li / Li +循环至4.5 V的石墨/ LiNi 0.6 Co 0.2 Mn 0.2 O 2电池的电化学性能。4.5 V循环结果表明,在50次循环后,当向基线电解液中添加1%CEP(1 M LiPF)时,石墨/ LiNi 0.6 Co 0.2 Mn 0.2 O 2电池的最高4.5 V容量保持率从27.4显着提高至81.5%。6在EC / EMC 1:2中,按重量计)。异位表征结果支持CEP增强电化学性能的机制。一方面,通过CEP在阴极电极表面上的优先氧化,抑制了高电压下的电解质分解,从而显着增强了形成的高级阴极界面膜。另一方面,二重路易斯碱官能团可有效捕获LiPF 6中的解离产物PF 5。电解液中不可避免地存在痕量的水或非质子杂质。因此,这减轻了氢氟酸(HF)的产生,氢氟酸的产生导致在高压下循环时电解质中过渡金属溶解的减少。理论模型表明,CEP具有通过-C≡N:官能团和H 2 O的组合来稳定电解质的机制。此处介绍的工作还显示了核磁共振光谱分析,以证明CEP减少HF生成和X-的能力。射线光电子能谱分析以观察阴极表面成分。
更新日期:2018-05-29
中文翻译:

通过操纵高压下石墨/ LiNi 0.6 Co 0.2 Mn 0.2 O 2电池电解质添加剂的官能团来构建独特的阴极界面
已经研究了一种新型的电解质添加剂1-(2-氰乙基)吡咯(CEP),以改善相对于Li / Li +循环至4.5 V的石墨/ LiNi 0.6 Co 0.2 Mn 0.2 O 2电池的电化学性能。4.5 V循环结果表明,在50次循环后,当向基线电解液中添加1%CEP(1 M LiPF)时,石墨/ LiNi 0.6 Co 0.2 Mn 0.2 O 2电池的最高4.5 V容量保持率从27.4显着提高至81.5%。6在EC / EMC 1:2中,按重量计)。异位表征结果支持CEP增强电化学性能的机制。一方面,通过CEP在阴极电极表面上的优先氧化,抑制了高电压下的电解质分解,从而显着增强了形成的高级阴极界面膜。另一方面,二重路易斯碱官能团可有效捕获LiPF 6中的解离产物PF 5。电解液中不可避免地存在痕量的水或非质子杂质。因此,这减轻了氢氟酸(HF)的产生,氢氟酸的产生导致在高压下循环时电解质中过渡金属溶解的减少。理论模型表明,CEP具有通过-C≡N:官能团和H 2 O的组合来稳定电解质的机制。此处介绍的工作还显示了核磁共振光谱分析,以证明CEP减少HF生成和X-的能力。射线光电子能谱分析以观察阴极表面成分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号