当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
HaloTag Assay Suggests Common Mechanism of E. coli Membrane Permeabilization Induced by Cationic Peptides
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acschembio.8b00336 Zhilin Yang 1 , James C. Weisshaar 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acschembio.8b00336 Zhilin Yang 1 , James C. Weisshaar 1
Affiliation
|
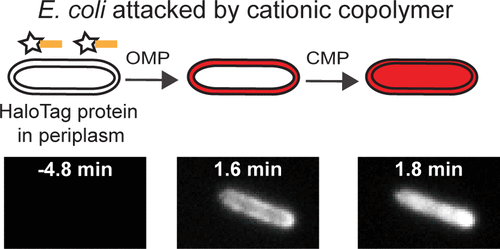
Permeabilization of the Gram-negative bacterial outer membrane (OM) by antimicrobial peptides (AMPs) is the initial step enabling access of the AMP to the cytoplasmic membrane. We present a new single-cell, time-resolved fluorescence microscopy assay that reports on the permeabilization of the E. coli OM to small molecules with a time resolution of 3 s or better. When profluorophore JF646 (702 Da) crosses the outer membrane (OM) and gains access to the periplasm, it binds to the localized HaloTag protein (34 kDa) and fluoresces in a characteristic hollow spatial pattern. Previous work used the much larger periplasmic GFP (27 kDa) probe, which reports on OM permeabilization to globular proteins. We test the assay on three cationic agents: Gellman random β–peptide copolymer MM63:CHx37, human AMP LL-37, and synthetic hybrid AMP CM15. These results combined with the previous work suggest a unifying sequence of OM and cytoplasmic membrane (CM) events that may prove commonplace in the attack of cationic peptides on Gram-negative bacteria. The peptide initially induces gradual OM permeabilization to small molecules, likely including the peptide itself. After a lag time, abrupt permeabilization of the OM, abrupt resealing of the OM, and abrupt permeabilization of the CM (all to globular proteins) occur in rapid sequence. We propose a mechanism based on membrane curvature stress induced by the time-dependent differential binding of peptide to the outer leaflet of the OM and CM. The results provide fresh insight into the critical OM-permeabilization step leading to a variety of damaging downstream events.
中文翻译:

HaloTag分析表明阳离子肽诱导的大肠杆菌膜透化的共同机制
抗菌肽(AMP)对革兰氏阴性细菌外膜(OM)的透化作用是使AMP进入细胞质膜的第一步。我们提出了一种新的单细胞,时间分辨荧光显微镜检测方法,该方法报告了大肠杆菌OM对小分子的通透性,其时间分辨率为3 s或更短。当前荧光团JF 646(702 Da)穿过外膜(OM)并进入周质时,它与局部HaloTag蛋白(34 kDa)结合并以特征性的中空空间模式发出荧光。先前的工作使用的是更大的周质GFP(27 kDa)探针,该探针报道了OM通透化为球状蛋白的情况。我们对三种阳离子试剂进行了测试:Gellman无规β肽共聚物MM63:CHx 37,人类AMP LL-37和合成混合动力AMP CM15。这些结果与先前的工作相结合,表明了OM和细胞质膜(CM)事件的统一序列,这在阳离子肽对革兰氏阴性细菌的攻击中可能是司空见惯的。肽最初会诱导OM逐渐渗透至小分子,可能包括肽本身。在滞后时间之后,OM突然变透,OM突然重新密封,CM突然变透(全部对球状蛋白)。我们提出了一种基于膜曲率应力的机制,该膜曲率是由肽与OM和CM的外部小叶的时间依赖性差异结合引起的。结果为关键的OM通透性步骤提供了新的见解,从而导致了各种破坏性的下游事件。
更新日期:2018-05-29
中文翻译:

HaloTag分析表明阳离子肽诱导的大肠杆菌膜透化的共同机制
抗菌肽(AMP)对革兰氏阴性细菌外膜(OM)的透化作用是使AMP进入细胞质膜的第一步。我们提出了一种新的单细胞,时间分辨荧光显微镜检测方法,该方法报告了大肠杆菌OM对小分子的通透性,其时间分辨率为3 s或更短。当前荧光团JF 646(702 Da)穿过外膜(OM)并进入周质时,它与局部HaloTag蛋白(34 kDa)结合并以特征性的中空空间模式发出荧光。先前的工作使用的是更大的周质GFP(27 kDa)探针,该探针报道了OM通透化为球状蛋白的情况。我们对三种阳离子试剂进行了测试:Gellman无规β肽共聚物MM63:CHx 37,人类AMP LL-37和合成混合动力AMP CM15。这些结果与先前的工作相结合,表明了OM和细胞质膜(CM)事件的统一序列,这在阳离子肽对革兰氏阴性细菌的攻击中可能是司空见惯的。肽最初会诱导OM逐渐渗透至小分子,可能包括肽本身。在滞后时间之后,OM突然变透,OM突然重新密封,CM突然变透(全部对球状蛋白)。我们提出了一种基于膜曲率应力的机制,该膜曲率是由肽与OM和CM的外部小叶的时间依赖性差异结合引起的。结果为关键的OM通透性步骤提供了新的见解,从而导致了各种破坏性的下游事件。



























 京公网安备 11010802027423号
京公网安备 11010802027423号