Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Alkylation of N–C Axially Chiral Quinazolinone Derivatives Bearing Various ortho-Substituted Phenyl Groups: Relation between Diastereoselectivity and the ortho-Substituent
Synlett ( IF 1.7 ) Pub Date : 2018-05-29 , DOI: 10.1055/s-0037-1610110 Osamu Kitagawa 1 , Mizuki Matsuoka 1 , Asumi Iida 1
Synlett ( IF 1.7 ) Pub Date : 2018-05-29 , DOI: 10.1055/s-0037-1610110 Osamu Kitagawa 1 , Mizuki Matsuoka 1 , Asumi Iida 1
Affiliation
|
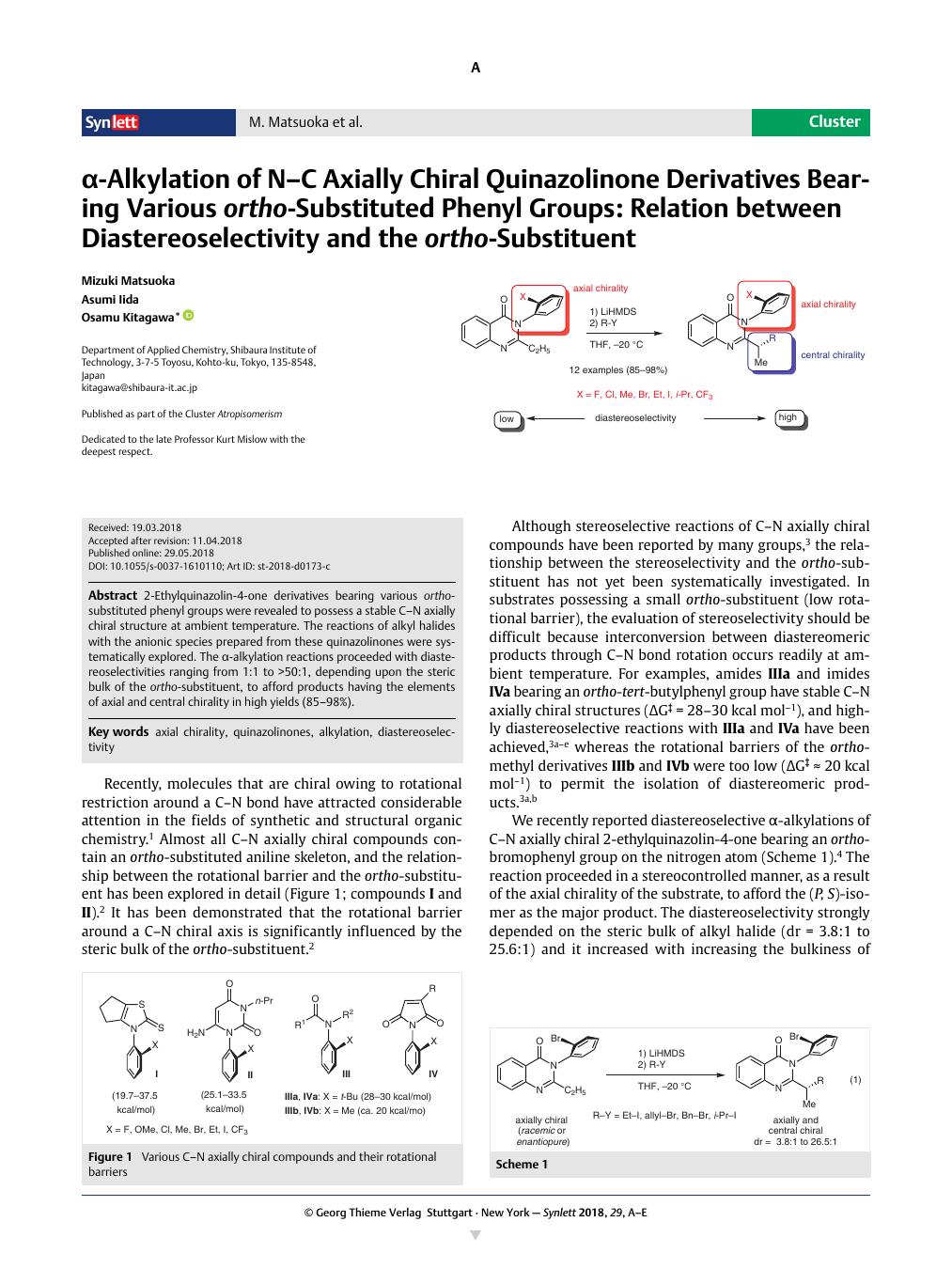
2-Ethylquinazolin-4-one derivatives bearing various ortho-substituted phenyl groups were revealed to possess a stable C–N axially chiral structure at ambient temperature. The reactions of alkyl halides with the anionic species prepared from these quinazolinones were systematically explored. The α-alkylation reactions proceeded with diastereoselectivities ranging from 1:1 to >50:1, depending upon the steric bulk of the ortho-substituent, to afford products having the elements of axial and central chirality in high yields (85–98%).
中文翻译:

带有各种邻位取代苯基的 N-C 轴向手性喹唑啉酮衍生物的 α-烷基化:非对映选择性与邻位取代基的关系
带有各种邻位取代苯基的 2-Ethylquinazolin-4-one 衍生物被证明在环境温度下具有稳定的 C-N 轴向手性结构。系统地研究了卤代烷与由这些喹唑啉酮制备的阴离子物质的反应。α-烷基化反应以 1:1 到 >50:1 的非对映选择性进行,这取决于邻位取代基的空间体积,以高产率(85-98%)提供具有轴和中心手性元素的产物.
更新日期:2018-05-29
中文翻译:

带有各种邻位取代苯基的 N-C 轴向手性喹唑啉酮衍生物的 α-烷基化:非对映选择性与邻位取代基的关系
带有各种邻位取代苯基的 2-Ethylquinazolin-4-one 衍生物被证明在环境温度下具有稳定的 C-N 轴向手性结构。系统地研究了卤代烷与由这些喹唑啉酮制备的阴离子物质的反应。α-烷基化反应以 1:1 到 >50:1 的非对映选择性进行,这取决于邻位取代基的空间体积,以高产率(85-98%)提供具有轴和中心手性元素的产物.











































 京公网安备 11010802027423号
京公网安备 11010802027423号