International Journal of Greenhouse Gas Control ( IF 4.6 ) Pub Date : 2018-05-28 , DOI: 10.1016/j.ijggc.2018.04.019 Kun Yu , Vignesh Peranamallur Rajan , Ning Dai
|
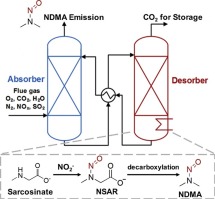
Amino acids are emerging as alternative solvents for absorption-based CO2 capture, but their potential to form the harmful byproducts nitrosamines is not well understood. This study investigated the formation of nitrosamines from model amino acids sarcosine, proline, and taurine under desorber- and absorber-relevant conditions. Special attention was paid to the effects of base and the formation of volatile nitrosamines. Under simulated desorber conditions, all three amino acid solvents with organic base monoethanolamine (MEA) formed more total nitrosamines than those with inorganic base sodium hydroxide (NaOH). Increasing base concentration decreased the formation of N-nitrososarcosine (NSAR) in both NaOH- and MEA-based sarcosine solvents. By measuring NSAR formation under varying base concentration and CO2 loading, it was shown that both sarcosine carbamic acid (SARCOOH) and deprotonated sarcosine (SAR−) contributed to NSAR formation. Volatile nitrosamine N-nitrosodimethylamine (NDMA) was detected in sarcosine solvents at levels three orders of magnitude lower than NSAR. Kinetic modeling showed that 61%–110% of the accumulated NDMA was formed through NSAR decomposition. NDMA formation from NSAR was promoted by increasing MEA concentration, but suppressed by increasing NaOH concentration. Under simulated absorber conditions, NaOH- and MEA-based solvents formed total nitrosamines at similar rates for sarcosine, proline, and taurine. In sarcosine solvents, NDMA formed at levels three orders of magnitude lower than NSAR, similar to that under desorber conditions.
中文翻译:

基于氨基酸的二氧化碳捕集系统中亚硝胺形成的动力学和途径
氨基酸正在作为基于吸收的CO 2捕集的替代溶剂而出现,但人们对形成有害副产物亚硝胺的潜力还知之甚少。这项研究调查了在脱附剂和吸收剂相关条件下由模型氨基酸肌氨酸,脯氨酸和牛磺酸形成的亚硝胺。特别注意了碱的影响和挥发性亚硝胺的形成。在模拟解吸器条件下,与有机碱一乙醇胺(MEA)相比,所有三种氨基酸溶剂形成的亚硝胺总量均高于无机碱氢氧化钠(NaOH)。增加碱浓度减少了氮的形成-亚硝基肌氨酸(NSAR)在基于NaOH和MEA的肌氨酸溶剂中。通过改变碱的浓度和CO下测量NSAR形成2加载,它表明,无论肌氨酸氨基甲酸(SARCOOH)和去质子化肌氨酸(SAR - )促成NSAR形成。挥发性亚硝胺N在肌氨酸溶剂中检测到亚硝基二甲胺(NDMA),其水平比NSAR低三个数量级。动力学建模表明,累积的NDMA的61%–110%是通过NSAR分解形成的。通过增加MEA浓度可促进由NSAR形成NDMA,但通过增加NaOH浓度可抑制NDMA的形成。在模拟吸收剂条件下,肌氨酸,脯氨酸和牛磺酸的NaOH和MEA基溶剂形成总亚硝胺的速率相似。在肌氨酸溶剂中,NDMA的形成水平比NSAR低三个数量级,这与解吸条件下的形成相似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号