当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Impurity Rejection Analysis for Crystallization
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-05-28 00:00:00 , DOI: 10.1021/acs.oprd.8b00143 Daniel D. Caspi 1 , Fredrik L. Nordstrom 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-05-28 00:00:00 , DOI: 10.1021/acs.oprd.8b00143 Daniel D. Caspi 1 , Fredrik L. Nordstrom 2
Affiliation
|
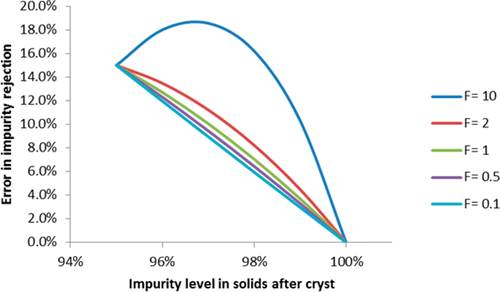
The analysis of impurity profiles is one of the most frequently performed activities in drug development. While mass-based quantification using a reference standard often provides the highest degree of accuracy, carrying this out on every impurity across the synthetic scheme can quickly become untenable when considering the often large number of impurities and the requirement for a synthesized and characterized reference standard. These labor-intensive activities are generally reserved for only selected impurities using a science- and risk-based approach, and approximations such as “purge factor” or “before and after peak area %” are often used in the remaining cases when a wider margin of error is acceptable. Presented herein is a simple mathematical framework to perform mass-based quantification for impurity rejection in silico using the principles of mass balance without the burdensome requirements of obtaining individual response factors or reference standards. This analysis is expected to provide useful guidance for process and analytical chemists.
中文翻译:

定量杂质排斥分析
杂质分布图分析是药物开发中最常执行的活动之一。尽管使用参考标准品进行基于质量的定量分析通常可以提供最高的准确性,但考虑到杂质通常很多以及对合成的和表征的参考标准品的要求,在合成方案中对每种杂质进行检测都将很快变得站不住脚。通常使用基于科学和风险的方法将这些劳动密集型活动仅保留给选定的杂质,并且在剩余情况下,当裕度较大时,通常使用近似值,例如“清除因子”或“峰面积%之前和之后”错误是可以接受的。本文介绍的是一个简单的数学框架,可使用质量平衡原理在计算机上对杂质的排除进行基于质量的定量,而无需获得单个响应因子或参考标准的繁重要求。该分析有望为工艺和分析化学家提供有用的指导。
更新日期:2018-05-28
中文翻译:

定量杂质排斥分析
杂质分布图分析是药物开发中最常执行的活动之一。尽管使用参考标准品进行基于质量的定量分析通常可以提供最高的准确性,但考虑到杂质通常很多以及对合成的和表征的参考标准品的要求,在合成方案中对每种杂质进行检测都将很快变得站不住脚。通常使用基于科学和风险的方法将这些劳动密集型活动仅保留给选定的杂质,并且在剩余情况下,当裕度较大时,通常使用近似值,例如“清除因子”或“峰面积%之前和之后”错误是可以接受的。本文介绍的是一个简单的数学框架,可使用质量平衡原理在计算机上对杂质的排除进行基于质量的定量,而无需获得单个响应因子或参考标准的繁重要求。该分析有望为工艺和分析化学家提供有用的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号