当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Large-bite diboranes for the μ(1,2) complexation of hydrazine and cyanide†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1039/c8sc01877d Chang-Hong Chen 1 , François P Gabbaï 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1039/c8sc01877d Chang-Hong Chen 1 , François P Gabbaï 1
Affiliation
|
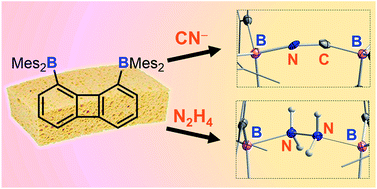
As part of our interest in the chemistry of polydentate Lewis acids as hosts for diatomic molecules, we have investigated the synthesis and coordination chemistry of bidentate boranes that feature a large boron–boron separation. In this paper, we describe the synthesis of a new example of such a diborane, namely 1,8-bis(dimesitylboryl)triptycene (2) and compare its properties to those of the recently reported 1,8-bis(dimesitylboryl)biphenylene (1). These comparative studies reveal that these two diboranes feature some important differences. As indicated by cyclic voltammetry, 1 is more electron deficient than 2; it also adopts a more compact and rigid structure with a boron–boron separation (4.566(5) Å) shorter by ∼1 Å than that in 2 (5.559(4) Å). These differences appear to dictate the coordination behaviour of these two compounds. While 2 remains inert toward hydrazine, we observed that 1 forms a very stable μ(1,2) hydrazine complex which can also be obtained by phase transfer upon layering a solution of 1 with a dilute aqueous hydrazine solution. The stability of this complex is further reflected by its lack of reaction with benzaldehyde at room temperature. We have also investigated the behaviour of 1 and 2 toward anions. In MeOH/CHCl3 (1/1 vol) both compounds selectively bind cyanide to form the corresponding μ(1,2) chelate complexes with a B–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–B bridge at their cores. Competition experiments in protic media show that the anionic cyanide complex formed by 1 is the most stable, with no evidence of decomplexation even in the presence of (C6F5)3B.
N–B bridge at their cores. Competition experiments in protic media show that the anionic cyanide complex formed by 1 is the most stable, with no evidence of decomplexation even in the presence of (C6F5)3B.
中文翻译:

用于肼和氰化物的 μ(1,2) 络合的大咬合乙硼烷†
作为我们对多齿路易斯酸作为双原子分子主体的化学兴趣的一部分,我们研究了具有大硼-硼分离特征的二齿硼烷的合成和配位化学。在本文中,我们描述了这种乙硼烷的新例子的合成,即 1,8-双(二甲基硼基)三蝶烯(2),并将其性质与最近报道的 1,8-双(二甲基硼基)联苯(1 )。这些比较研究表明,这两种乙硼烷具有一些重要的差异。如循环伏安法所示,1比2更缺电子;它还采用了更紧凑和刚性的结构,硼 - 硼分离(4.566(5)Å)比在2(5.559(4)埃)。这些差异似乎决定了这两种化合物的配位行为。虽然2对肼保持惰性,但我们观察到1形成非常稳定的 μ(1,2) 肼络合物,该络合物也可以通过将1的溶液与稀肼水溶液分层后的相转移来获得。该复合物的稳定性进一步反映在它在室温下不与苯甲醛反应。我们还研究了1和2对阴离子的行为。在 MeOH/CHCl 3 (1/1 vol) 中,这两种化合物选择性地结合氰化物以形成相应的 μ(1,2) 螯合物与 B–C![[三键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N-B 桥在它们的核心。在质子介质中的竞争实验表明,由1形成的阴离子氰化物络合物是最稳定的,即使在 (C 6 F 5 ) 3 B存在下也没有解络合的迹象。
N-B 桥在它们的核心。在质子介质中的竞争实验表明,由1形成的阴离子氰化物络合物是最稳定的,即使在 (C 6 F 5 ) 3 B存在下也没有解络合的迹象。
更新日期:2018-05-29
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–B bridge at their cores. Competition experiments in protic media show that the anionic cyanide complex formed by 1 is the most stable, with no evidence of decomplexation even in the presence of (C6F5)3B.
N–B bridge at their cores. Competition experiments in protic media show that the anionic cyanide complex formed by 1 is the most stable, with no evidence of decomplexation even in the presence of (C6F5)3B.
中文翻译:

用于肼和氰化物的 μ(1,2) 络合的大咬合乙硼烷†
作为我们对多齿路易斯酸作为双原子分子主体的化学兴趣的一部分,我们研究了具有大硼-硼分离特征的二齿硼烷的合成和配位化学。在本文中,我们描述了这种乙硼烷的新例子的合成,即 1,8-双(二甲基硼基)三蝶烯(2),并将其性质与最近报道的 1,8-双(二甲基硼基)联苯(1 )。这些比较研究表明,这两种乙硼烷具有一些重要的差异。如循环伏安法所示,1比2更缺电子;它还采用了更紧凑和刚性的结构,硼 - 硼分离(4.566(5)Å)比在2(5.559(4)埃)。这些差异似乎决定了这两种化合物的配位行为。虽然2对肼保持惰性,但我们观察到1形成非常稳定的 μ(1,2) 肼络合物,该络合物也可以通过将1的溶液与稀肼水溶液分层后的相转移来获得。该复合物的稳定性进一步反映在它在室温下不与苯甲醛反应。我们还研究了1和2对阴离子的行为。在 MeOH/CHCl 3 (1/1 vol) 中,这两种化合物选择性地结合氰化物以形成相应的 μ(1,2) 螯合物与 B–C
![[三键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N-B 桥在它们的核心。在质子介质中的竞争实验表明,由1形成的阴离子氰化物络合物是最稳定的,即使在 (C 6 F 5 ) 3 B存在下也没有解络合的迹象。
N-B 桥在它们的核心。在质子介质中的竞争实验表明,由1形成的阴离子氰化物络合物是最稳定的,即使在 (C 6 F 5 ) 3 B存在下也没有解络合的迹象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号