当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into the SN2-type reactivity of aryl-Co(iii) masked-carbenes for C–C bond forming transformations†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1039/c8sc00851e O Planas 1 , S Roldán-Gómez 1 , V Martin-Diaconescu 2 , J M Luis 1 , A Company 1 , X Ribas 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1039/c8sc00851e O Planas 1 , S Roldán-Gómez 1 , V Martin-Diaconescu 2 , J M Luis 1 , A Company 1 , X Ribas 1
Affiliation
|
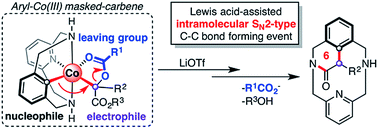
Herein we describe the synthesis and characterization of a family of C-metalated aryl-Co(III) enolates, which can be considered as masked-carbenes, using diazoacetates as coupling partners. These species have been proved to be necessary intermediates in the C(sp2)–C(sp3) bond forming event to obtain cyclic amides, taming the elusive Co(III)-carbene species. The scope of diazoacetates has been exhaustively examined, varying the nature of the ester and the α-substitution, and a clear preference for electron-poor carbene precursors is observed. Exhaustive experimental and theoretical studies indicate that an unprecedented intramolecular SN2-type process governs the formation of the newly formed C–C bond. Furthermore, the key role of several Lewis acids as carboxylate-activating reagents is further explored by DFT calculations.
中文翻译:

芳基-Co(iii)掩蔽卡宾的SN2型反应性对C-C键形成转化的机理见解†
在此,我们描述了C-金属化芳基-Co( III )烯醇化物家族的合成和表征,其可以被视为掩蔽卡宾,使用重氮乙酸酯作为偶联配偶体。这些物质已被证明是 C(sp 2 )–C(sp 3 ) 键形成过程中获得环酰胺的必要中间体,从而驯服了难以捉摸的 Co( III )-卡宾物质。重氮乙酸酯的范围已经被详尽地研究,改变了酯和α-取代的性质,并且观察到对缺电子卡宾前体的明显偏好。详尽的实验和理论研究表明,前所未有的分子内 S N 2 型过程控制着新形成的 C-C 键的形成。此外,通过 DFT 计算进一步探讨了几种路易斯酸作为羧酸盐活化剂的关键作用。
更新日期:2018-05-29
中文翻译:

芳基-Co(iii)掩蔽卡宾的SN2型反应性对C-C键形成转化的机理见解†
在此,我们描述了C-金属化芳基-Co( III )烯醇化物家族的合成和表征,其可以被视为掩蔽卡宾,使用重氮乙酸酯作为偶联配偶体。这些物质已被证明是 C(sp 2 )–C(sp 3 ) 键形成过程中获得环酰胺的必要中间体,从而驯服了难以捉摸的 Co( III )-卡宾物质。重氮乙酸酯的范围已经被详尽地研究,改变了酯和α-取代的性质,并且观察到对缺电子卡宾前体的明显偏好。详尽的实验和理论研究表明,前所未有的分子内 S N 2 型过程控制着新形成的 C-C 键的形成。此外,通过 DFT 计算进一步探讨了几种路易斯酸作为羧酸盐活化剂的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号