Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-05-28 , DOI: 10.1016/j.bmcl.2018.05.051 Kosei Yamauchi , Akari Fujieda , Tohru Mitsunaga
|
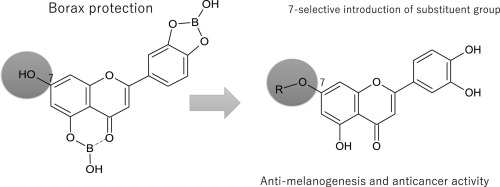
In our previous study, the isolation of ugonin J, K, and L, which are luteolin derivatives, from the roots of Helminthostachys zeylanica and their identification as potent melanogenesis inhibitors, was described. The structure activity relationship (SAR) investigation in that study revealed that the catechol moiety in the B-ring of the flavone skeleton of ugonin K was important for its melanogenesis inhibitory activity, and the presence of the low polarity substituents at the C-7 position enhanced this activity. In order to further investigate the SAR of the C-7-substituent in the luteolin derivatives, different groups were selectively introduced at the C-7 position of luteolin after borax protection of the catechol hydroxyl group and the C-5 hydroxyl group. NMR and MS analysis of the borax protected derivatives revealed that the borax protects not only hydroxyl groups of catechol on the B ring but also the 5-hydroxyl group on the A ring. Eight luteolin derivatives were synthesized and evaluated for melanogenesis inhibitory effect in B16 melanoma cells. Two bulky groups and six alkoxyl groups were introduced at the C-7 position. The resulting luteolin derivatives showed improved melanogenesis and cell proliferation inhibitory activities. From among these derivatives, 7-O-hexylluteolin (7) showed the highest activity and inhibited the melanogenesis to 14% at 6.25 μM. The present study also revealed that the length of the carbon chain rather than the bulky substituent was more important for the melanogenesis inhibitory activity.
中文翻译:

B16黑色素瘤细胞中7- O-取代木犀草素衍生物的选择性合成及其对黑素的抑制和增殖抑制活性
在我们以前的研究中,ugonin J,K,和L上的隔离,这是木犀草素衍生物,从根部七指蕨并描述了它们作为有效的黑色素生成抑制剂的鉴定。这项研究中的结构活性关系(SAR)研究表明,ugonin K的黄酮骨架B环中的邻苯二酚部分对其抑制黑色素生成活性以及C-7位置存在低极性取代基很重要。增强了这项活动。为了进一步研究木犀草素衍生物中C-7取代基的SAR,在硼砂保护邻苯二酚羟基和C-5羟基后,将不同的基团选择性地引入木犀草素的C-7位置。硼砂保护的衍生物的NMR和MS分析表明,硼砂不仅保护B环上邻苯二酚的羟基,而且保护A环上的5-羟基。合成了八种木犀草素衍生物,并评估了其对B16黑色素瘤细胞中黑色素生成的抑制作用。在C-7位引入两个大的基团和六个烷氧基。所得木犀草素衍生物显示出改善的黑色素生成和细胞增殖抑制活性。在这些衍生物中,7-O-己基木犀草素(7)在6.25μM时表现出最高的活性并将黑色素生成抑制到14%。本研究还表明,碳链的长度而不是庞大的取代基对于黑色素生成抑制活性更为重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号