当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Proteoids Generated From Dipeptide‐Based Monomers
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2018-05-28 , DOI: 10.1002/marc.201800099 Yun Liu 1 , Marc C. A. Stuart 2 , Eric Buhler 3 , Anna K. H. Hirsch 4, 5
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2018-05-28 , DOI: 10.1002/marc.201800099 Yun Liu 1 , Marc C. A. Stuart 2 , Eric Buhler 3 , Anna K. H. Hirsch 4, 5
Affiliation
|
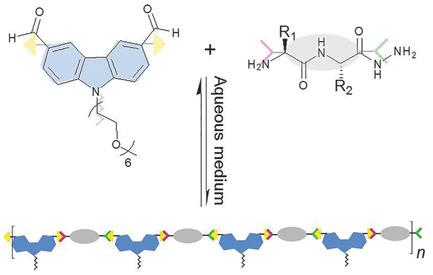
Dynamic proteoids are dynamic covalent analogues of proteins which are generated through the reversible polymerization of amino‐acid‐ or peptide‐derived monomers. The authors design and prepare a series of dynamic proteoids based on the reversible polycondensation of six types of dipeptide hydrazides bearing different categories of side chains. The polymerization and structures of biodynamers generated by 1H‐NMR spectroscopy, light scattering and cryo‐transmission‐electron microscopy are studied. This study shows that the presence of aromatic rings in the side chains plays the most essential role in determining the extent of the polymerization and organization into resultant nanostructures through π−π‐stacking interactions, hydroxyl groups have a less favorable influence via hydrogen bonds, whereas a high density of positive charge blocks the generation of biodynamers due to electrostatic repulsions. These findings set the stage for the rational design and synthesis of dynamic proteoids as novel biofunctional materials.
中文翻译:

由基于二肽的单体生成的动态蛋白
动态蛋白是蛋白质的动态共价类似物,它是通过氨基酸或肽衍生的单体的可逆聚合反应生成的。作者基于六种带有不同侧链类别的二肽酰肼的可逆缩聚,设计和制备了一系列动态蛋白。1产生的生物名称的聚合和结构研究了H-NMR光谱,光散射和低温透射电子显微镜。这项研究表明,侧链中芳环的存在在决定聚合程度和通过π-π堆积相互作用形成纳米结构中起着至关重要的作用,羟基通过氢键的影响较小。由于静电排斥,高密度的正电荷会阻止生物染料的产生。这些发现为合理设计和合成动态蛋白作为新型生物功能材料奠定了基础。
更新日期:2018-05-28
中文翻译:

由基于二肽的单体生成的动态蛋白
动态蛋白是蛋白质的动态共价类似物,它是通过氨基酸或肽衍生的单体的可逆聚合反应生成的。作者基于六种带有不同侧链类别的二肽酰肼的可逆缩聚,设计和制备了一系列动态蛋白。1产生的生物名称的聚合和结构研究了H-NMR光谱,光散射和低温透射电子显微镜。这项研究表明,侧链中芳环的存在在决定聚合程度和通过π-π堆积相互作用形成纳米结构中起着至关重要的作用,羟基通过氢键的影响较小。由于静电排斥,高密度的正电荷会阻止生物染料的产生。这些发现为合理设计和合成动态蛋白作为新型生物功能材料奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号