当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Synthetic Methodologies via Catalytic Enantioselective Synthesis of 3,3-Disubstituted Oxindoles
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.accounts.8b00097 Zhong-Yan Cao , Feng Zhou , Jian Zhou 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-05-29 00:00:00 , DOI: 10.1021/acs.accounts.8b00097 Zhong-Yan Cao , Feng Zhou , Jian Zhou 1
Affiliation
|
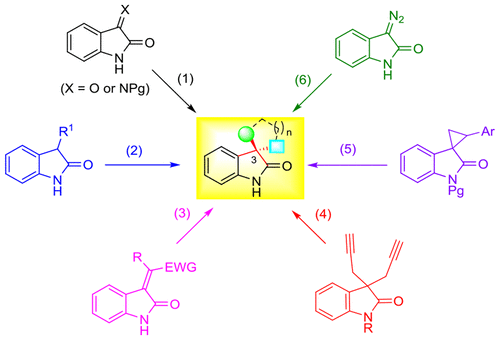
3,3-Disubstituted oxindoles are widely distributed in natural products, drugs, and pharmaceutically active compounds. The absolute configuration and the substituents on the fully substituted C3 stereocenter of the oxindole often significantly influence the biological activity. Therefore, tremendous efforts have made to develop catalytic enantioselective syntheses of this prominent structural motif. Research in this area is further fueled by the ever-increasing demand for modern probe- and drug-discovery programs for synthetic libraries of chiral compounds that are derived from privileged scaffolds with high structural diversity. Notably, the efficient construction of fully substituted C3 stereocenters of oxindole, tetrasubstituted or all-carbon quaternary, spirocyclic or not, also becomes a test ground for new synthetic methodologies.
中文翻译:

通过催化对映选择性合成3,3-二取代的羟吲哚合成方法的发展
3,3-二取代的羟吲哚广泛分布于天然产物,药物和药物活性化合物中。羟吲哚的完全取代的C3立体中心上的绝对构型和取代基通常会显着影响生物活性。因此,付出了巨大的努力来开发这种突出的结构基序的催化对映选择性合成。对现代探针和药物发现程序的日益增长的需求进一步推动了这一领域的发展,这些程序用于从具有高结构多样性的特权支架中衍生出来的手性化合物的合成文库。值得注意的是,高效取代的四取代或全碳取代的四取代或全碳取代的羟吲哚的完全取代的C3立体中心,也成为新合成方法的试验场。
更新日期:2018-05-29
中文翻译:

通过催化对映选择性合成3,3-二取代的羟吲哚合成方法的发展
3,3-二取代的羟吲哚广泛分布于天然产物,药物和药物活性化合物中。羟吲哚的完全取代的C3立体中心上的绝对构型和取代基通常会显着影响生物活性。因此,付出了巨大的努力来开发这种突出的结构基序的催化对映选择性合成。对现代探针和药物发现程序的日益增长的需求进一步推动了这一领域的发展,这些程序用于从具有高结构多样性的特权支架中衍生出来的手性化合物的合成文库。值得注意的是,高效取代的四取代或全碳取代的四取代或全碳取代的羟吲哚的完全取代的C3立体中心,也成为新合成方法的试验场。











































 京公网安备 11010802027423号
京公网安备 11010802027423号