当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective [3 + 2] cycloaddition and rearrangement of thiazolium salts to synthesize thiazole and 1,4-thiazine derivatives†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-28 00:00:00 , DOI: 10.1039/c8qo00370j Xiying Zhang 1, 2, 3, 4, 5 , Xiaohua Liu 1, 2, 3, 4, 5 , Jianlin Zhang 1, 2, 3, 4, 5 , Dong Zhang 1, 2, 3, 4, 5 , Lili Lin 1, 2, 3, 4, 5 , Xiaoming Feng 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-28 00:00:00 , DOI: 10.1039/c8qo00370j Xiying Zhang 1, 2, 3, 4, 5 , Xiaohua Liu 1, 2, 3, 4, 5 , Jianlin Zhang 1, 2, 3, 4, 5 , Dong Zhang 1, 2, 3, 4, 5 , Lili Lin 1, 2, 3, 4, 5 , Xiaoming Feng 1, 2, 3, 4, 5
Affiliation
|
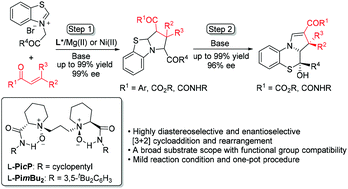
Highly efficient asymmetric [3 + 2] cycloaddition reactions of thiazolium salts with a variety of electron-deficient 2-π components were achieved by chiral N,N′-dioxide/metal complex catalysts, providing a convenient route to access enantioenriched hydropyrrolo-thiazoles. The oxathiazine and sulfinate could be readily obtained by the oxidation of the hydropyrrolo-thiazole. Moreover, through ingenious collaboration of chiral catalysts with basic additives, the first cascade cycloaddition/rearrangement reaction with β,γ-unsaturated α-ketoesters was realized, and various pyrrolo-[1,4]thiazine derivatives were afforded in excellent results.
中文翻译:

噻唑盐的对映选择性[3 + 2]环加成和重排,以合成噻唑和1,4-噻嗪衍生物†
通过手性N,N'-二氧化物/金属络合物催化剂,可实现噻唑鎓盐与多种电子不足的2-π组分的高效不对称[3 + 2]环加成反应,为获得对映体富集的氢吡咯并噻唑提供了便利的途径。氧杂吡咯并噻唑的氧化可容易地获得草并噻嗪和亚磺酸盐。此外,通过手性催化剂与碱性添加剂的巧妙配合,实现了与β,γ-不饱和α-酮酸酯的第一级联环加成/重排反应,并得到了各种吡咯-[1,4]噻嗪衍生物。
更新日期:2018-05-28
中文翻译:

噻唑盐的对映选择性[3 + 2]环加成和重排,以合成噻唑和1,4-噻嗪衍生物†
通过手性N,N'-二氧化物/金属络合物催化剂,可实现噻唑鎓盐与多种电子不足的2-π组分的高效不对称[3 + 2]环加成反应,为获得对映体富集的氢吡咯并噻唑提供了便利的途径。氧杂吡咯并噻唑的氧化可容易地获得草并噻嗪和亚磺酸盐。此外,通过手性催化剂与碱性添加剂的巧妙配合,实现了与β,γ-不饱和α-酮酸酯的第一级联环加成/重排反应,并得到了各种吡咯-[1,4]噻嗪衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号