Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-05-26 , DOI: 10.1016/j.bioorg.2018.05.024 Tao Wu , Ping He , Wei Wu , Yingli Chen , Fenglin Lv
|
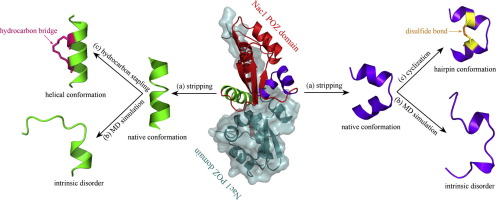
The oncogenic transcriptional corepressor Nac1 contains a conserved POZ protein–protein interaction module that mediates homodimerization or heterodimerization with itself or other POZ proteins. The dimerization has been recognized as an attractive target for cancer therapy. Here, we attempted to (i) discover those potential binding partners of Nac1 in the human genome, (ii) derive key peptide segments from the complex interface of Nac1 with its putative partners, and (iii) improve the peptide binding affinity to Nac1 POZ domain. In the procedure, Nac1 POZ dimerization with 136 human POZ domains was modeled, simulated and analyzed at atomic level to elucidate structural basis, energetic property and dynamics behavior. Two hotspot regions, namely α1-helix and α2/α3-hairpin, at the dimerization interface were identified that are responsible for stabilizing the formed POZ–POZ dimer complexes. The α1-helix and α2/α3-hairpin were stripped from the interface to derive their respective isolated SIP peptides, which, however, exhibited a large flexibility and intrinsic disorder in free state, and thus would incur a considerable penalty upon rebinding to Nac1 POZ domain. By carefully examining the natively folded structures of α1-helix and α2/α3-hairpin in protein context and their interaction modes with the domain, we rationally designed a hydrocarbon bridge and a disulfide bond separately for the two peptides in order to constrain their conformational flexibility in free state, thus largely minimizing the flexibility penalty. Consequently, three α1-helix peptides derived from Nac1, Miz1 and Slx4 were stapled by all-hydrocarbon bridge, while four α2/α3-hairpin peptides derived from Nac1, Bacd1, Klh28 and Mynn were cyclized by disulfide bond. Binding affinity analysis revealed that, as designed, these peptides were converted from non- or weak binders to moderate or good binders of Nac1 POZ domain upon the stapling and cyclization.
中文翻译:

通过环化和装订靶向靶向构象受限肽的致癌转录共抑制因子Nac1 POZ域
致癌转录共抑制因子Nac1包含一个保守的POZ蛋白-蛋白相互作用模块,该模块介导自身或其他POZ蛋白的同二聚或异二聚。二聚化已被认为是癌症治疗的有吸引力的靶标。在这里,我们试图(i)在人类基因组中发现Nac1的那些潜在结合配体,(ii)从Nac1与其推定的配体的复杂界面中获得关键的肽段,以及(iii)改善与Nac1 POZ的肽结合亲和力领域。在该程序中,在原子水平上对具有136个人类POZ域的Nac1 POZ二聚化进行了建模,模拟和分析,以阐明结构基础,能量性质和动力学行为。两个热点区域,即α1-螺旋和α2/α3-发夹,在二聚化界面处鉴定出负责稳定形成的POZ-POZ二聚体复合物的分子。从界面上剥离了α1-螺旋和α2/α3-发夹,以得到它们各自的分离的SIP肽,但是,它们在自由状态下表现出很大的柔韧性和内在无序性,因此在重新结合至Nac1 POZ时会遭受可观的损失。领域。通过仔细检查蛋白质环境中α1-螺旋和α2/α3-发夹的天然折叠结构及其与结构域的相互作用模式,我们合理地设计了两种肽的烃桥和二硫键,以限制它们的构象柔性在自由状态下,因此大大降低了灵活性损失。因此,衍生自Nac1的三个α1-螺旋肽 Miz1和Slx4被全烃桥钉住,而衍生自Nac1,Bacd1,Klh28和Mynn的四个α2/α3-发夹肽通过二硫键环化。结合亲和力分析显示,按照设计,这些肽在装订和环化后从非结合剂或弱结合剂转变为Nac1 POZ域的中等或良好结合剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号