Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2018-05-25 , DOI: 10.1016/j.jcou.2018.05.018 Pooya Lahijani , Maedeh Mohammadi , Abdul Rahman Mohamed
|
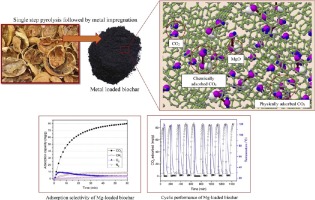
This work investigates the development of metal incorporated biochar as a low-cost and robust adsorbent for high capacity CO2 capture at ambient condition. For this purpose, biochars were prepared through single-step pyrolysis of walnut shell (WS) at different temperatures (500, 700 and 900 °C). The biochar with larger specific surface area and higher microporosity fraction (WS900) was then subjected to metal impregnation followed by N2 heat treatment. The incorporation of basic metal sites into the biochar skeleton enhanced the adsorption of acidic CO2 gas onto the metalized-biochar in the sequence of Mg > Al > Fe > Ni > Ca > raw-biochar > Na. The enhanced CO2 uptake of Mg-loaded biochar (82.0 mg/g) compared to the pristine biochar (72.6 mg/g) at 25 °C and 1 atm, could be explained by the synergistic effects of physical and chemical interactions, yet kinetic studies showed that physisorption was the main governing mechanism controlling the adsorption of CO2 onto the metalized-biochar. Cyclic CO2 capture studies indicated the great stability of Mg-loaded biochar upon several cycles of adsorption-desorption, with no loss in its CO2 capture capacity. It also showed easy regeneration and fast desorption kinetic which makes it a promising inexpensive candidate for real-world CO2 capture systems. Moreover, the adsorbent showed a superior capture performance towards CO2 over N2, O2 and CH4.
中文翻译:

金属结合的生物炭作为潜在的吸附剂,可在环境条件下捕获高容量的CO 2
这项工作研究了掺入金属的生物炭作为一种低成本,坚固的吸附剂在环境条件下捕获高容量CO 2的开发。为此,通过在不同温度(500、700和900°C)下对核桃壳(WS)进行单步热解来制备生物炭。然后,将具有较大的比表面积和较高的微孔率(WS900)的生物炭进行金属浸渍,然后进行N 2热处理。碱性金属位点进入生物炭骨架中增强了酸性CO 2气体在金属化生物炭上的吸附顺序,依次为Mg> Al> Fe> Ni> Ca>原始生物炭> Na。增强的CO 2在25°C和1个大气压下,与原始生物炭(72.6 mg / g)相比,载有Mg的生物炭(82.0 mg / g)的吸收可以通过物理和化学相互作用的协同作用来解释,但动力学研究表明,物理吸附是控制CO 2在金属化生物炭上的吸附的主要控制机制。循环CO 2捕集研究表明,在几个吸附-解吸循环后,负载镁的生物炭具有很大的稳定性,而其CO 2捕集能力没有损失。它还显示出容易的再生和快速的解吸动力学,这使其成为现实世界中CO 2捕集系统的有希望的廉价候选者。此外,该吸附剂对CO 2的捕获性能优异。在N 2,O 2和CH 4上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号