当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Density Functional Theory Investigation of the Role of Cocatalytic Water in Methane Steam Reforming over Anatase TiO2 (101)
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2018-06-08 , DOI: 10.1021/acs.iecr.8b00944 Alec Hook , Timothy P. Nuber , Fuat E. Celik
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2018-06-08 , DOI: 10.1021/acs.iecr.8b00944 Alec Hook , Timothy P. Nuber , Fuat E. Celik
|
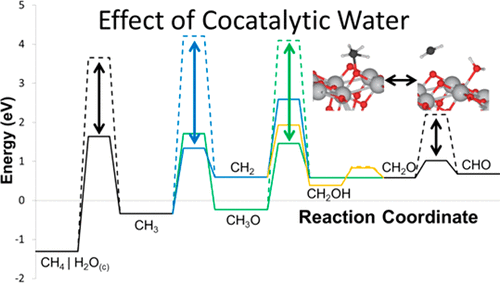
The methane steam reforming (MSR) mechanism on the anatase TiO2 (101) surface has been investigated using periodic density functional theory. Reaction energies for several pathways were calculated, and pathways involving high energy intermediates were eliminated from consideration. The remaining pathways all involved formaldehyde and formyl as intermediates. Activation energy barriers were calculated for these, revealing that C–H activation in methane and elementary dehydrogenation reactions in general involved very high energy transition states when calculated on clean surfaces. When water was coadsorbed on reaction surfaces as chemisorbed OH and H, hydrogen transfer reactions were facilitated by the proximity between hydrogen donor and acceptor and the strong affinity of hydroxyl for hydrogen. As the product of such hydrogen transfer reactions was physisorbed water, water activation completes the catalytic cycle, and the role of water can be termed cocatalytic. Cocatalytic water lowered the most difficult dehydrogenation barriers by 2–3 eV, including reducing the methane activation barrier from 4.96 to 2.95 eV, making activity on the TiO2 surface relevant at high MSR reaction temperatures. Because of the high barrier for CHO dehydrogenation, the water-gas shift reaction is expected to make CO2 the preferred product, rather than CO, during MSR over metal-free TiO2.
更新日期:2018-06-09



























 京公网安备 11010802027423号
京公网安备 11010802027423号