当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of the Lysine Acylomes and the Substrates Regulated by Protein Acyltransferase in Mycobacterium smegmatis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1021/acschembio.8b00213 Jun-Yu Xu 1, 2 , Lei Zhao 1 , XinXin Liu 1 , Hao Hu 2 , Ping Liu 2 , Minjia Tan 2 , Bang-Ce Ye 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1021/acschembio.8b00213 Jun-Yu Xu 1, 2 , Lei Zhao 1 , XinXin Liu 1 , Hao Hu 2 , Ping Liu 2 , Minjia Tan 2 , Bang-Ce Ye 1
Affiliation
|
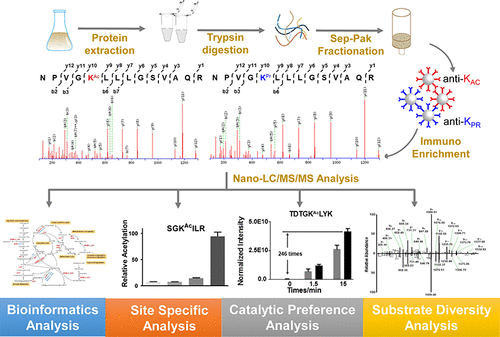
Protein acylation plays important roles in bacterial pathogenesis through regulation of enzymatic activity, protein stability, nucleic acid binding ability, and protein–protein interactions. Mycobacteria, a genus including invasive pathogens known to cause serious diseases, shapes its pathogenicity through adaptation of its energy metabolism to microenvironments encountered within mammalian hosts. In this process, acetyl-CoA and propionyl-CoA function as important intermediates. However, the function of acetyl-CoA/propionyl-CoA driven protein acylation remains to be elucidated. Herein, we systematically investigated protein acetylome/propionylome in the nonpathogenic Mycobacterium smegmatis through antibody-enrichment-based proteomic analysis in which 146 acetylated sites on 121 proteins and 26 propionylated sites on 25 proteins were identified. After that, characteristic differences of the two acylomes were elucidated through such bioinformatic methods as motif analysis, protein–protein analysis, Gene Ontology analysis, and KEGG analysis. In addition, quantitative mass spectrometric method was used to evaluate the site-specific and motif-biased catalytic mechanism mediated by the cAMP-dependent acetyltransferase MsKat in M. smegmatis. Furthermore, we raised the possibility that both O-serine and Nε-lysine acetylation might coregulate the propionyl-CoA synthetase. This study described the landscape of acetylome and propionylome in the M. smegmatis, showing an unexpected role of protein acylation regulation in mycobacteria.
中文翻译:

耻垢分枝杆菌中赖氨酸的酰基转移酶和蛋白质酰基转移酶调控的底物的表征
蛋白质酰化通过调节酶活性,蛋白质稳定性,核酸结合能力和蛋白质-蛋白质相互作用,在细菌发病中起重要作用。分枝杆菌属,包括已知会引起严重疾病的侵入性病原体,是通过使其能量代谢适应哺乳动物宿主内所遇到的微环境而改变其致病性的。在此过程中,乙酰辅酶A和丙酰辅酶A作为重要的中间体。然而,乙酰辅酶A /丙酰辅酶A驱动的蛋白酰化的功能仍有待阐明。在这里,我们系统地研究了非致病性耻垢分枝杆菌中的蛋白质乙酰基/丙酰基组。通过基于抗体富集的蛋白质组分析,鉴定了121个蛋白质上的146个乙酰化位点和25个蛋白质上的26个丙酰化位点。此后,通过基序分析,蛋白质-蛋白质分析,基因本体分析和KEGG分析等生物信息学方法阐明了两个突的特征差异。此外,定量质谱法用于评估由耻垢分枝杆菌中的cAMP依赖性乙酰基转移酶Ms Kat介导的位点特异性和基序偏向催化机制。此外,我们上调的可能,这两个Ø丝氨酸和ñ ε-赖氨酸的乙酰化作用可能使丙酰辅酶A合成酶形成核心。这项研究描述了耻垢分枝杆菌中的乙酰组和丙组的景观,显示了分枝杆菌中蛋白质酰化调控的意想不到的作用。
更新日期:2018-05-25
中文翻译:

耻垢分枝杆菌中赖氨酸的酰基转移酶和蛋白质酰基转移酶调控的底物的表征
蛋白质酰化通过调节酶活性,蛋白质稳定性,核酸结合能力和蛋白质-蛋白质相互作用,在细菌发病中起重要作用。分枝杆菌属,包括已知会引起严重疾病的侵入性病原体,是通过使其能量代谢适应哺乳动物宿主内所遇到的微环境而改变其致病性的。在此过程中,乙酰辅酶A和丙酰辅酶A作为重要的中间体。然而,乙酰辅酶A /丙酰辅酶A驱动的蛋白酰化的功能仍有待阐明。在这里,我们系统地研究了非致病性耻垢分枝杆菌中的蛋白质乙酰基/丙酰基组。通过基于抗体富集的蛋白质组分析,鉴定了121个蛋白质上的146个乙酰化位点和25个蛋白质上的26个丙酰化位点。此后,通过基序分析,蛋白质-蛋白质分析,基因本体分析和KEGG分析等生物信息学方法阐明了两个突的特征差异。此外,定量质谱法用于评估由耻垢分枝杆菌中的cAMP依赖性乙酰基转移酶Ms Kat介导的位点特异性和基序偏向催化机制。此外,我们上调的可能,这两个Ø丝氨酸和ñ ε-赖氨酸的乙酰化作用可能使丙酰辅酶A合成酶形成核心。这项研究描述了耻垢分枝杆菌中的乙酰组和丙组的景观,显示了分枝杆菌中蛋白质酰化调控的意想不到的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号