Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-05-26 , DOI: 10.1016/j.actbio.2018.05.044 Dianjun Qi , Shaohua Wu , Mitchell A. Kuss , Wen Shi , Soonkyu Chung , Paul T. Deegan , Alexey Kamenskiy , Yini He , Bin Duan
|
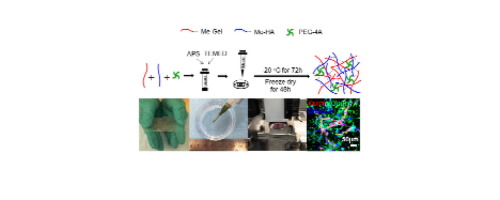
Bioengineered adipose tissues have gained increased interest as a promising alternative to autologous tissue flaps and synthetic adipose fillers for soft tissue augmentation and defect reconstruction in clinic. Although many scaffolding materials and biofabrication methods have been investigated for adipose tissue engineering in the last decades, there are still challenges to recapitulate the appropriate adipose tissue microenvironment, maintain volume stability, and induce vascularization to achieve long-term function and integration. In the present research, we fabricated cryogels consisting of methacrylated gelatin, methacrylated hyaluronic acid, and 4arm poly(ethylene glycol) acrylate (PEG-4A) by using cryopolymerization. The cryogels were repeatedly injectable and stretchable, and the addition of PEG-4A improved the robustness and mechanical properties. The cryogels supported human adipose progenitor cell (HWA) and adipose derived mesenchymal stromal cell adhesion, proliferation, and adipogenic differentiation and maturation, regardless of the addition of PEG-4A. The HWA laden cryogels facilitated the co-culture of human umbilical vein endothelial cells (HUVEC) and capillary-like network formation, which in return also promoted adipogenesis. We further combined cryogels with 3D bioprinting to generate handleable adipose constructs with clinically relevant size. 3D bioprinting enabled the deposition of multiple bioinks onto the cryogels. The bioprinted flap-like constructs had an integrated structure without delamination and supported vascularization.
Significance
Adipose tissue engineering is promising for reconstruction of soft tissue defects, and also challenging for restoring and maintaining soft tissue volume and shape, and achieving vascularization and integration. In this study, we fabricated cryogels with mechanical robustness, injectability, and stretchability by using cryopolymerization. The cryogels promoted cell adhesion, proliferation, and adipogenic differentiation and maturation of human adipose progenitor cells and adipose derived mesenchymal stromal cells. Moreover, the cryogels also supported 3D bioprinting on top, forming vascularized adipose constructs. This study demonstrates the potential of the implementation of cryogels for generating volume-stable adipose tissue constructs and provides a strategy to fabricate vascularized flap-like constructs for complex soft tissue regeneration.
中文翻译:

机械坚固的冷冻凝胶,具有可注射性和生物印刷支持性,可用于脂肪组织工程
生物工程化的脂肪组织作为自体组织瓣和合成脂肪填充剂在临床上用于软组织增强和缺损重建的一种有前途的替代方案,引起了越来越多的关注。尽管在过去的几十年中,已经针对脂肪组织工程研究了许多脚手架材料和生物制造方法,但是仍然存在挑战,以概括合适的脂肪组织微环境,维持体积稳定性并诱导血管形成以实现长期功能和整合。在本研究中,我们通过冷冻聚合制备了由甲基丙烯酸酯化的明胶,甲基丙烯酸酯化的透明质酸和4臂聚(乙二醇)丙烯酸酯(PEG-4A)组成的冷冻凝胶。冰晶石可反复注射和拉伸,PEG-4A的加入改善了坚固性和机械性能。冷冻凝胶支持人脂肪祖细胞(HWA)和脂肪来源的间充质基质细胞的粘附,增殖以及成脂分化和成熟,无论是否加入PEG-4A。富含HWA的冷冻凝胶促进了人脐静脉内皮细胞(HUVEC)的共培养和毛细血管状网络的形成,这反过来也促进了脂肪形成。我们进一步将冷冻凝胶与3D生物打印相结合,以生成具有临床相关大小的可处理的脂肪构建体。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。冷冻凝胶支持人脂肪祖细胞(HWA)和脂肪来源的间充质基质细胞的粘附,增殖以及成脂分化和成熟,无论是否加入PEG-4A。富含HWA的冷冻凝胶促进了人脐静脉内皮细胞(HUVEC)的共培养和毛细血管状网络的形成,这反过来也促进了脂肪形成。我们进一步将冷冻凝胶与3D生物打印相结合,以生成具有临床相关大小的可处理的脂肪构建体。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。冷冻凝胶支持人脂肪祖细胞(HWA)和脂肪来源的间充质基质细胞的粘附,增殖以及成脂分化和成熟,无论是否加入PEG-4A。富含HWA的冷冻凝胶促进了人脐静脉内皮细胞(HUVEC)的共培养和毛细血管状网络的形成,这反过来也促进了脂肪形成。我们进一步将冷冻凝胶与3D生物打印相结合,以生成具有临床相关大小的可处理的脂肪构建体。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。不管加入PEG-4A如何。富含HWA的冷冻凝胶促进了人脐静脉内皮细胞(HUVEC)的共培养和毛细血管状网络的形成,这反过来也促进了脂肪形成。我们进一步将冷冻凝胶与3D生物打印相结合,以生成具有临床相关大小的可处理的脂肪构建体。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。不管加入PEG-4A如何。富含HWA的冷冻凝胶促进了人脐静脉内皮细胞(HUVEC)的共培养和毛细血管状网络的形成,这反过来也促进了脂肪形成。我们进一步将冷冻凝胶与3D生物打印相结合,以生成具有临床相关大小的可处理的脂肪构建体。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。3D生物打印使多种生物墨水能够沉积在冰晶上。生物印制的瓣状构建体具有完整的结构,没有分层,并支持了血管形成。
意义
脂肪组织工程技术有望重建软组织缺损,并且对于恢复和维持软组织的体积和形状以及实现血管形成和整合也具有挑战性。在这项研究中,我们通过使用冷冻聚合工艺制造了具有机械坚固性,可注射性和可拉伸性的冰冻凝胶。冷冻凝胶促进人脂肪祖细胞和脂肪来源的间充质基质细胞的细胞粘附,增殖以及成脂分化和成熟。此外,冷冻凝胶还支持在顶部进行3D生物打印,形成带血管的脂肪结构。这项研究证明了冷冻凝胶产生潜在的稳定体积的脂肪组织构建体的潜力,并提供了制造用于复杂软组织再生的血管化皮瓣样构建体的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号