Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-05-26 , DOI: 10.1016/j.actbio.2018.05.041 Jungho Ahn , Chong-Su Cho , Seong Woo Cho , Joo H. Kang , Sung-Yon Kim , Dal-Hee Min , Joon Myong Song , Tae-Eun Park , Noo Li Jeon
|
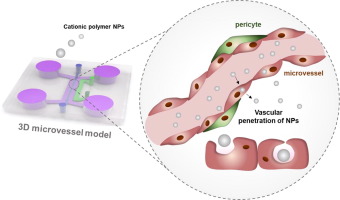
Vascular networks are the first sites exposed to cationic polymer nanoparticles (NPs) administered intravenously, and thus function as a barrier for NPs reaching the target organ. While cationic polymer NPs have been intensively studied as non-viral delivery systems, their biological effects in human microvessels have been poorly investigated due to a lack of appropriate in vitro systems. Here, we employed a three-dimensional microvessel on a chip, which accurately models in vivo conditions. An open and perfused microvessel surrounded by pericytes was shown to reproduce the important features of living vasculature, including barrier function and biomarkers. Using this microvessel chip, we observed contraction of the microvascular lumen induced by perfused polyethylenimine (PEI)/DNA NPs. We demonstrated that the oxidative stress present when microvessels were exposed to PEI NPs led to rearrangement of microtubules resulting in microvessel contraction. Furthermore, the transcytotic behavior of PEI NPs was analyzed in the microvessel by monitoring the escape of PEI NPs from the microvascular lumen into the perivascular region, which was not possible in two-dimensional culture systems. With our new understanding of the different behaviors of cationic polymer NPs depending on their transcytotic route, we suggest that caveolae-mediated transcytosis is a powerful route for efficient extravascular transport.
The statement of significance
Microvascular networks are not only biological system constituting largest surface area in the body and but also first site exposed to nanoparticle in vivo. While cationic polymer NPs have been intensively studied as non-viral delivery systems, its biological effects in human microvessel have been poorly investigated due to lack of appropriate in vitro systems. Here, we microengineered an open and perfused 3D pericyte incorporated microvessel model which possesses same morphological characteristic of in vivo. Using the microengineered model, this study represents the first report of transcytotic behavior of NPs in 3D microvessel, and its effect on extravasation efficiency. Our study lays the groundwork for the integration of innovative technologies to examine blood vessel-nanoparticle interaction, which a critical but ill-defined phenomenon.
中文翻译:

使用可灌注3D微血管模型研究阳离子聚合物纳米颗粒的血管细胞毒性和血管外运输
血管网络是暴露于静脉内施用的阳离子聚合物纳米颗粒(NPs)的第一个位点,因此可作为NPs到达靶器官的屏障。尽管已经对阳离子聚合物NPs作为非病毒递送系统进行了深入研究,但由于缺乏合适的体外系统,人们对其阳离子在人体微血管中的生物学作用进行了较差的研究。在这里,我们在芯片上采用了三维微血管,可以在体内准确地建模条件。显示一个被周细胞包围的开放的灌注微血管可再现活脉管系统的重要特征,包括屏障功能和生物标志物。使用该微血管芯片,我们观察到由灌注的聚乙烯亚胺(PEI)/ DNA NPs诱导的微血管腔收缩。我们证明了当微血管暴露于PEI NPs时存在的氧化应激会导致微管的重排,从而导致微血管收缩。此外,通过监测PEI NPs从微血管腔进入血管周围区域的逃逸来分析PEI NPs在微血管中的跨细胞行为,这在二维培养系统中是不可能的。根据我们对阳离子聚合物NP跨细胞途径的不同行为的新认识,
重要声明
微血管网络不仅是构成人体最大表面积的生物系统,而且也是体内暴露于纳米粒子的第一个部位。尽管已经将阳离子聚合物NPs作为非病毒递送系统进行了深入研究,但由于缺乏合适的体外系统,人们对其阳离子在人微血管中的生物学作用的研究还很少。在这里,我们微工程了一个开放的和灌注的3D周细胞并入的微血管模型,该模型具有与体内相同的形态学特征。使用微工程模型,这项研究代表了3D微血管中NPs的胞吞行为及其对外渗效率的影响的首次报道。我们的研究为整合创新技术以检查血管-纳米颗粒的相互作用奠定了基础,这是一个关键但不确定的现象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号