当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2-aminobenzaldehydes by rhodium(iii)-catalyzed C–H amidation of aldehydes with dioxazolones†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8qo00413g Chen-Fei Liu 1, 2, 3, 4, 5 , Man Liu 1, 2, 3, 4, 5 , Jun-Shu Sun 1, 2, 3, 4, 5 , Chao Li 1, 2, 3, 4, 5 , Lin Dong 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8qo00413g Chen-Fei Liu 1, 2, 3, 4, 5 , Man Liu 1, 2, 3, 4, 5 , Jun-Shu Sun 1, 2, 3, 4, 5 , Chao Li 1, 2, 3, 4, 5 , Lin Dong 1, 2, 3, 4, 5
Affiliation
|
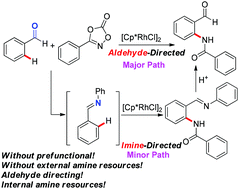
Various aldehydes were directly amidated with dioxazolones to afford 2-aminobenzaldehydes through rhodium(III)-catalyzed CHO-directed C–H activation. Aldehydes as the directing groups can effectively minimize waste formation and pre-functionalization steps. Notably, another pathway through which the traceless imine directing groups are generated by a self-reaction between benzaldehydes and dioxazolones has also been proposed in the catalytic system. Further synthetic application of 2-aminobenzaldehydes can lead to more attractive heterocyclic frameworks.
中文翻译:

铑(iii)催化醛与二恶唑酮的CH–H酰胺化反应合成2-氨基苯甲醛†
各种醛直接与二恶唑酮酰胺化,通过铑(III)催化的CHO定向C–H活化得到2-氨基苯甲醛。醛作为导向基团可以有效地减少废物形成和预功能化步骤。值得注意的是,在催化体系中还提出了另一种途径,通过该途径通过苯甲醛和二恶唑酮之间的自反应产生无痕的亚胺导向基团。2-氨基苯甲醛的进一步合成应用可导致更具吸引力的杂环骨架。
更新日期:2018-05-25
中文翻译:

铑(iii)催化醛与二恶唑酮的CH–H酰胺化反应合成2-氨基苯甲醛†
各种醛直接与二恶唑酮酰胺化,通过铑(III)催化的CHO定向C–H活化得到2-氨基苯甲醛。醛作为导向基团可以有效地减少废物形成和预功能化步骤。值得注意的是,在催化体系中还提出了另一种途径,通过该途径通过苯甲醛和二恶唑酮之间的自反应产生无痕的亚胺导向基团。2-氨基苯甲醛的进一步合成应用可导致更具吸引力的杂环骨架。









































 京公网安备 11010802027423号
京公网安备 11010802027423号