当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly enantioselective Friedel–Crafts alkylation of N,N-dialkylanilines with trans-β-nitrostyrene catalyzed by a homochiral metal–organic framework†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8cc03447h Koichi Tanaka 1, 2, 3, 4, 5 , Kenji Sakuragi 1, 2, 3, 4, 5 , Hiroto Ozaki 1, 2, 3, 4, 5 , Yoshiki Takada 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8cc03447h Koichi Tanaka 1, 2, 3, 4, 5 , Kenji Sakuragi 1, 2, 3, 4, 5 , Hiroto Ozaki 1, 2, 3, 4, 5 , Yoshiki Takada 1, 2, 3, 4, 5
Affiliation
|
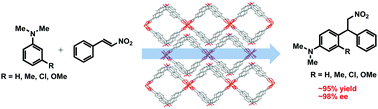
The first enantioselective Friedel–Crafts alkylation of N,N-dialkylanilines with trans-β-nitrostyrene catalyzed by a homochiral metal–organic framework constructed from (R)-2,2′-dihydroxy-1,1′-binaphthyl-6,6′-dicarboxylic acid has been developed. The reaction proceeded in high yields (∼96%) with excellent enantioselectivities (∼98% ee).
中文翻译:

的高度对映选择性的Friedel-Crafts烷基化Ñ,Ñ -dialkylanilines与反式由纯手性金属-有机构架催化-β硝基苯乙烯†
(R)-2,2'-dihydroxy-1,1'-binaphthyl-6,6(6,6)构筑的手性金属-有机骨架催化的N,N-二烷基苯胺与反式-β-硝基苯乙烯的首次对映选择性Friedel-Crafts烷基化反应已经开发了'-二羧酸。反应以高产率(〜96%)和优异的对映选择性(〜98%ee)进行。
更新日期:2018-05-25
中文翻译:

的高度对映选择性的Friedel-Crafts烷基化Ñ,Ñ -dialkylanilines与反式由纯手性金属-有机构架催化-β硝基苯乙烯†
(R)-2,2'-dihydroxy-1,1'-binaphthyl-6,6(6,6)构筑的手性金属-有机骨架催化的N,N-二烷基苯胺与反式-β-硝基苯乙烯的首次对映选择性Friedel-Crafts烷基化反应已经开发了'-二羧酸。反应以高产率(〜96%)和优异的对映选择性(〜98%ee)进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号