当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable syngas production from photocatalytic CO2 reduction with mitigated charge recombination driven by spatially separated cocatalysts†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8sc01812j Ang Li 1 , Tuo Wang 1 , Xiaoxia Chang 1 , Zhi-Jian Zhao 1 , Chengcheng Li 1 , Zhiqi Huang 1 , Piaoping Yang 1 , Guangye Zhou 1 , Jinlong Gong 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8sc01812j Ang Li 1 , Tuo Wang 1 , Xiaoxia Chang 1 , Zhi-Jian Zhao 1 , Chengcheng Li 1 , Zhiqi Huang 1 , Piaoping Yang 1 , Guangye Zhou 1 , Jinlong Gong 1
Affiliation
|
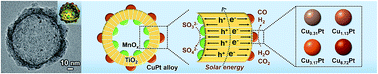
Photocatalytic CO2 reduction represents a sustainable route to generate syngas (the mixture of CO and H2), which is a key feedstock to produce liquid fuels in industry. Yet this reaction typically suffers from two limitations: unsuitable CO/H2 ratio and serious charge recombination. This paper describes the production of syngas from photocatalytic CO2 reduction with a tunable CO/H2 ratio via adjustment of the components and surface structure of CuPt alloys and construction of a TiO2 mesoporous hollow sphere with spatially separated cocatalysts to promote charge separation. Unlike previously reported cocatalyst-separated hollow structures, we firstly create a reductive outer surface that is suitable for the CO2 reduction reaction. A high evolution rate of 84.2 μmol h−1 g−1 for CO and a desirable CO/H2 ratio of 1 : 2 are achieved. The overall solar energy conversion yield is 0.108%, which is higher than those of traditional oxide and sulfide based catalysts (generally about 0.006–0.042%). Finally, density functional theory calculations and kinetic experiments by replacing H2O with D2O reveal that the enhanced activity is mainly determined by the reduction energy of CO* and can be affected by the stability of COOH*.
中文翻译:

通过光催化二氧化碳还原产生可调节的合成气,并通过空间分离的助催化剂驱动减轻电荷重组†
光催化CO 2还原是产生合成气(CO 和H 2的混合物)的可持续途径,合成气是工业生产液体燃料的关键原料。然而,该反应通常受到两个限制:不合适的CO/H 2比例和严重的电荷重组。本文描述了通过调整CuPt合金的成分和表面结构以及构建具有空间分离的助催化剂以促进电荷分离的TiO 2介孔空心球,以可调的CO/H 2比例通过光催化CO 2还原生产合成气。与之前报道的助催化剂分离的中空结构不同,我们首先创建适合CO 2还原反应的还原性外表面。实现了84.2 μmol h -1 g -1的高CO释放速率和1:2的理想CO/H 2比率。太阳能总转化率为0.108%,高于传统氧化物和硫化物基催化剂(一般约为0.006-0.042%)。最后,密度泛函理论计算和用D 2 O代替H 2 O的动力学实验表明,活性的增强主要由CO*的还原能决定,并可能受到COOH*稳定性的影响。
更新日期:2018-05-25
中文翻译:

通过光催化二氧化碳还原产生可调节的合成气,并通过空间分离的助催化剂驱动减轻电荷重组†
光催化CO 2还原是产生合成气(CO 和H 2的混合物)的可持续途径,合成气是工业生产液体燃料的关键原料。然而,该反应通常受到两个限制:不合适的CO/H 2比例和严重的电荷重组。本文描述了通过调整CuPt合金的成分和表面结构以及构建具有空间分离的助催化剂以促进电荷分离的TiO 2介孔空心球,以可调的CO/H 2比例通过光催化CO 2还原生产合成气。与之前报道的助催化剂分离的中空结构不同,我们首先创建适合CO 2还原反应的还原性外表面。实现了84.2 μmol h -1 g -1的高CO释放速率和1:2的理想CO/H 2比率。太阳能总转化率为0.108%,高于传统氧化物和硫化物基催化剂(一般约为0.006-0.042%)。最后,密度泛函理论计算和用D 2 O代替H 2 O的动力学实验表明,活性的增强主要由CO*的还原能决定,并可能受到COOH*稳定性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号