当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2‐Acetamido‐2‐deoxy‐l‐iminosugar C‐Alkyl and C‐Aryl Glycosides: Synthesis and Glycosidase Inhibition
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-08-14 , DOI: 10.1002/ejoc.201800678 Nathalie Fontelle 1 , Arisa Yamamoto 2 , Ana Arda 3 , Jesús Jiménez-Barbero 3 , Atsushi Kato 2 , Jérôme Désiré 1 , Yves Blériot 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-08-14 , DOI: 10.1002/ejoc.201800678 Nathalie Fontelle 1 , Arisa Yamamoto 2 , Ana Arda 3 , Jesús Jiménez-Barbero 3 , Atsushi Kato 2 , Jérôme Désiré 1 , Yves Blériot 1
Affiliation
|
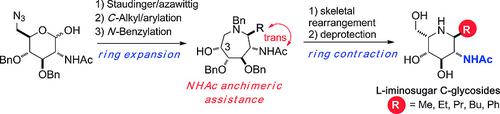
A Staudinger/azawittig/Grignard/ring‐contraction sequence applied to two d‐glycopyranose derivatives bearing an acetamido group at the C‐2 position and an azido group at the C‐6 position allows access to unprecedented six‐membered l‐iminosugars C‐glycosides as GlcNAc and ManNAc mimics. The high 1,2‐trans diastereoselectivity observed for the Grignard addition strongly suggests an anchimeric assistance by the NHAc group.

中文翻译:

2-乙酰胺基-2-脱氧-1-亚氨基糖C-烷基和C-芳基糖苷:合成和糖苷酶抑制
施陶丁格/ azawittig /格利雅/环收缩序列应用于两个d -glycopyranose衍生物轴承在C-2位乙酰氨基和叠氮基的C-6位置允许访问前所未有六元升-iminosugars Ç - GlcNAc和ManNAc模拟糖苷。格利雅(Grignard)添加物具有较高的1,2-反式非对映选择性,这强烈暗示了NHAc基团的对映体辅助作用。

更新日期:2018-08-14

中文翻译:

2-乙酰胺基-2-脱氧-1-亚氨基糖C-烷基和C-芳基糖苷:合成和糖苷酶抑制
施陶丁格/ azawittig /格利雅/环收缩序列应用于两个d -glycopyranose衍生物轴承在C-2位乙酰氨基和叠氮基的C-6位置允许访问前所未有六元升-iminosugars Ç - GlcNAc和ManNAc模拟糖苷。格利雅(Grignard)添加物具有较高的1,2-反式非对映选择性,这强烈暗示了NHAc基团的对映体辅助作用。












































 京公网安备 11010802027423号
京公网安备 11010802027423号