当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Properties That Define the Activities of Antibiotics in Escherichia coli and Pseudomonas aeruginosa
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1021/acsinfecdis.8b00036 Connor J Cooper 1 , Ganesh Krishnamoorthy 2 , David Wolloscheck 2 , John K Walker 3 , Valentin V Rybenkov 2 , Jerry M Parks 1, 4 , Helen I Zgurskaya 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1021/acsinfecdis.8b00036 Connor J Cooper 1 , Ganesh Krishnamoorthy 2 , David Wolloscheck 2 , John K Walker 3 , Valentin V Rybenkov 2 , Jerry M Parks 1, 4 , Helen I Zgurskaya 2
Affiliation
|
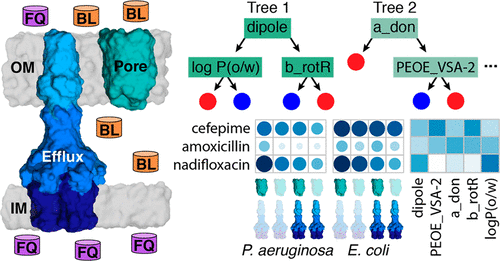
The permeability barrier of Gram-negative cell envelopes is the major obstacle in the discovery and development of new antibiotics. In Gram-negative bacteria, these difficulties are exacerbated by the synergistic interaction between two biochemically distinct phenomena, the low permeability of the outer membrane (OM) and active multidrug efflux. In this study, we used Pseudomonas aeruginosa and Escherichia coli strains with controllable permeability barriers, achieved through hyperporination of the OMs and varied efflux capacities, to evaluate the contributions of each of the barriers to protection from antibacterials. We analyzed antibacterial activities of β-lactams and fluoroquinolones, antibiotics that are optimized for targets in the periplasm and the cytoplasm, respectively, and performed a machine learning-based analysis to identify physicochemical descriptors that best classify their relative potencies. Our results show that the molecular properties selected by active efflux and the OM barriers are different for the two species. Antibiotic activity in P. aeruginosa was better classified by electrostatic and surface area properties, whereas topology, physical properties, and atom or bond counts best capture the behavior in E. coli. In several cases, descriptor values that correspond to active antibiotics also correspond to significant barrier effects, highlighting the synergy between the two barriers where optimizing for one barrier promotes strengthening of the other barrier. Thus, both barriers should be considered when optimizing antibiotics for favorable OM permeability, efflux evasion, or both.
中文翻译:

定义大肠杆菌和铜绿假单胞菌中抗生素活性的分子特性
革兰氏阴性细胞包膜的通透性障碍是发现和开发新抗生素的主要障碍。在革兰氏阴性细菌中,两个生化独特现象,外膜(OM)的低渗透性和活性多药外排之间的协同相互作用加剧了这些困难。在这项研究中,我们使用了铜绿假单胞菌和大肠杆菌通过OM的超孔化和不同的流出能力获得具有可控渗透性屏障的菌株,以评估每种屏障对抗菌药物的保护作用。我们分析了β-内酰胺类和氟喹诺酮类(分别针对周质和细胞质中的靶标进行了优化的抗生素)的抗菌活性,并进行了基于机器学习的分析,以鉴定能最佳分类其相对效价的理化指标。我们的研究结果表明,通过主动外排和OM屏障选择的分子性质对于这两个物种是不同的。铜绿假单胞菌的抗生素活性通过静电和表面积特性可以更好地进行分类,而拓扑结构,物理特性以及原子数或键数最能反映大肠杆菌的行为。在某些情况下,对应于活性抗生素的描述符值也对应于显着的屏障效应,突出了两种屏障之间的协同作用,其中针对一种屏障的优化促进了另一种屏障的强化。因此,在优化抗生素以获得良好的OM渗透性,外排逃逸性或两者兼而有之时,应同时考虑这两个障碍。
更新日期:2018-05-14
中文翻译:

定义大肠杆菌和铜绿假单胞菌中抗生素活性的分子特性
革兰氏阴性细胞包膜的通透性障碍是发现和开发新抗生素的主要障碍。在革兰氏阴性细菌中,两个生化独特现象,外膜(OM)的低渗透性和活性多药外排之间的协同相互作用加剧了这些困难。在这项研究中,我们使用了铜绿假单胞菌和大肠杆菌通过OM的超孔化和不同的流出能力获得具有可控渗透性屏障的菌株,以评估每种屏障对抗菌药物的保护作用。我们分析了β-内酰胺类和氟喹诺酮类(分别针对周质和细胞质中的靶标进行了优化的抗生素)的抗菌活性,并进行了基于机器学习的分析,以鉴定能最佳分类其相对效价的理化指标。我们的研究结果表明,通过主动外排和OM屏障选择的分子性质对于这两个物种是不同的。铜绿假单胞菌的抗生素活性通过静电和表面积特性可以更好地进行分类,而拓扑结构,物理特性以及原子数或键数最能反映大肠杆菌的行为。在某些情况下,对应于活性抗生素的描述符值也对应于显着的屏障效应,突出了两种屏障之间的协同作用,其中针对一种屏障的优化促进了另一种屏障的强化。因此,在优化抗生素以获得良好的OM渗透性,外排逃逸性或两者兼而有之时,应同时考虑这两个障碍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号