当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hetero-assembly of a dual β-amyloid variant peptide system†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8cc02724b Jason Candreva 1, 2, 3, 4 , Edward Chau 1, 2, 3, 4 , Edwin Aoraha 1, 2, 3, 4 , Vikas Nanda 4, 5, 6, 7 , Jin Ryoun Kim 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8cc02724b Jason Candreva 1, 2, 3, 4 , Edward Chau 1, 2, 3, 4 , Edwin Aoraha 1, 2, 3, 4 , Vikas Nanda 4, 5, 6, 7 , Jin Ryoun Kim 1, 2, 3, 4
Affiliation
|
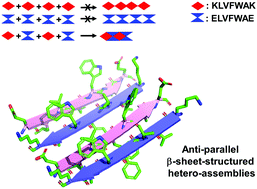
Self-assembly of amyloid polypeptides (1) imparts biological effects depending on the size in over 20 amyloid diseases and (2) produces useful yet relatively untapped biomaterials. Unfortunately, our understanding of amyloid polypeptides, as related to biomedical implications and biomaterial applications, is limited by their self-assembling nature. In this study, we report the creation of a dual peptide system, where a pair of β-amyloid (Aβ) variants are not self-assembled but hetero-assembled in the presence of their assembly partners. We provide evidence that the resulting hetero-assemblies share molecular, structural and morphological similarities with typical amyloid self-assemblies formed by a single polypeptide (e.g., Aβ). We anticipate that our dual peptide system may readily be adapted for precise control of amyloid assembly, for the study of size-dependent neurotoxicity and precise fabrication of amyloid biomaterials.
中文翻译:

双β-淀粉样变体肽系统的异源组装†
淀粉样蛋白多肽的自组装(1)根据20多种淀粉样蛋白疾病的大小赋予生物学效应,(2)生产有用但尚未开发的生物材料。不幸的是,我们对淀粉样多肽的了解与生物医学意义和生物材料应用有关,受到其自组装性质的限制。在这项研究中,我们报告了双肽系统的创建,其中一对β-淀粉样蛋白(Aβ)变体不是自组装的,而是在存在其组装伙伴的情况下是异组装的。我们提供了证据,即所得到的杂组件共享与由单一的多肽形成的淀粉样蛋白的典型自组装分子,结构和形态相似性(例如,Aβ)。我们预计,我们的双肽系统可以很容易地适用于淀粉样蛋白组装的精确控制,用于研究大小依赖性神经毒性和淀粉样生物材料的精确制造。
更新日期:2018-05-25
中文翻译:

双β-淀粉样变体肽系统的异源组装†
淀粉样蛋白多肽的自组装(1)根据20多种淀粉样蛋白疾病的大小赋予生物学效应,(2)生产有用但尚未开发的生物材料。不幸的是,我们对淀粉样多肽的了解与生物医学意义和生物材料应用有关,受到其自组装性质的限制。在这项研究中,我们报告了双肽系统的创建,其中一对β-淀粉样蛋白(Aβ)变体不是自组装的,而是在存在其组装伙伴的情况下是异组装的。我们提供了证据,即所得到的杂组件共享与由单一的多肽形成的淀粉样蛋白的典型自组装分子,结构和形态相似性(例如,Aβ)。我们预计,我们的双肽系统可以很容易地适用于淀粉样蛋白组装的精确控制,用于研究大小依赖性神经毒性和淀粉样生物材料的精确制造。



























 京公网安备 11010802027423号
京公网安备 11010802027423号