当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autoinductive conversion of α,α-diiodonitroalkanes to amides and esters catalysed by iodine byproducts under O2†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8cc03191f Jing Li 1, 2, 3, 4, 5 , Martin J. Lear 6, 7, 8, 9, 10 , Yujiro Hayashi 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-05-25 00:00:00 , DOI: 10.1039/c8cc03191f Jing Li 1, 2, 3, 4, 5 , Martin J. Lear 6, 7, 8, 9, 10 , Yujiro Hayashi 1, 2, 3, 4, 5
Affiliation
|
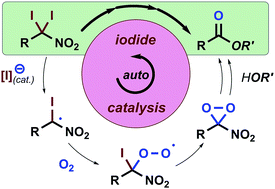
Studies to convert nitroalkanes into amides and esters using I2 and O2 revealed in situ-generated iodine species facilitate the homolytic C–I bond cleavage of α,α-diiodonitroalkanes, arguably in an autoinductive or autocatalytic manner. Consequently, we devised a rapid and economical I2/O2-based method to synthesise sterically hindered esters directly from primary nitroalkanes.
中文翻译:

在O 2 †下,碘副产物将α,α-二碘硝基烷烃自动感应转化为酰胺和酯。
使用I 2和O 2将硝基烷烃转化为酰胺和酯的研究表明,原位生成的碘可促进α,α-二碘硝基烷烃的均质C–I键裂解,可以说是自感应或自催化方式。因此,我们设计了一种快速且经济的基于I 2 / O 2的方法,直接从伯硝基烷烃中合成位阻酯。
更新日期:2018-05-25
中文翻译:

在O 2 †下,碘副产物将α,α-二碘硝基烷烃自动感应转化为酰胺和酯。
使用I 2和O 2将硝基烷烃转化为酰胺和酯的研究表明,原位生成的碘可促进α,α-二碘硝基烷烃的均质C–I键裂解,可以说是自感应或自催化方式。因此,我们设计了一种快速且经济的基于I 2 / O 2的方法,直接从伯硝基烷烃中合成位阻酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号