Tetrahedron ( IF 2.1 ) Pub Date : 2018-05-24 , DOI: 10.1016/j.tet.2018.05.068 Timothy J. Blackburn , Eric J. Thomas
|
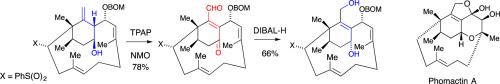
Previous studies of a synthesis of phomactin A had resulted in the synthesis of a 15-methylenebicyclo[9.3.1]pentadecadiene. The next step in the synthesis was to be the epoxidation of this methylenecyclohexane that was hoped would lead to a 1-(hydroxymethyl)cyclohexene by rearrangement of the exocyclic epoxide, but the epoxidation was difficult to carry out regioselectively on advanced intermediates. However, oxidation of a 15-methylenebicyclo[9.3.1]pentadeca-3,7-dien-14-ol using tetra-n-propylammonium perruthenate and N-methylmorpholine-N-oxide led to conversion of this homoallylic alcohol into the corresponding 14-oxobicyclo[9.3.1]pentadeca-1(15),3,7-triene-15-carboxaldehyde in one step. Reduction of this using DIBAL-H gave a promising intermediate for a synthesis of a phomactin. The scope of this oxidation of homoallylic alcohols was briefly investigated.
中文翻译:

苯羟肌动蛋白的合成方法:使用过钌酸四正丙铵对均丙醇进行新型氧化
以前对光蛋白A合成的研究已经合成了15-亚甲基双环[9.3.1]十五碳二烯。合成的下一步是该亚甲基环己烷的环氧化,希望通过环外环氧化物的重排生成1-(羟甲基)环己烯,但是环氧化很难在高级中间体上进行区域选择性。然而,使用过钌酸四正丙铵和N-甲基吗啉-N氧化15-亚甲基双环[9.3.1] pentadeca-3,7-dien-14-ol氧化物导致一步反应就将这种均烯丙基醇转化为相应的14-氧代双环[9.3.1] pentadeca-1(15),3,7-三烯-15-甲醛。用DIBAL-H还原得到的有希望的中间体可用于合成phomactin。简要研究了均烯丙基醇的这种氧化范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号