当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Diels–Alder Reaction/[3,3] Sigmatropic Rearrangement Cascade of 1‐Thiocyanatobutadienes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804811 Yuhang Zhou 1 , Lili Lin 1 , Xiaohua Liu 1 , Xinyue Hu 1 , Yan Lu 1 , Xiying Zhang 1 , Xiaoming Feng 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804811 Yuhang Zhou 1 , Lili Lin 1 , Xiaohua Liu 1 , Xinyue Hu 1 , Yan Lu 1 , Xiying Zhang 1 , Xiaoming Feng 1, 2
Affiliation
|
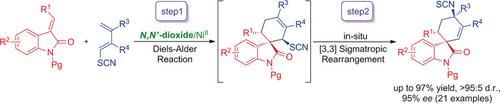
A highly efficient asymmetric Diels–Alder/[3,3] sigmatropic rearrangement reaction of methyleneindolinones with 1‐thiocyanatobutadienes has been realized by using a chiral N,N′‐dioxide/nickel(II) complex as the catalyst. A range of cyclohexenyl isothiocyanates were synthesized in high yields with excellent diastereo‐ and enantioselectivities. Based on mechanistic studies, a catalytic cycle with possible transition‐state models were proposed to explain the process.
中文翻译:

催化不对称Diels–Alder反应/ [1-硫氰酸根合丁二烯的[3,3]σ重排级联
通过使用手性N,N'-二氧化物/镍(II)配合物实现了亚甲基吲哚酮与1-硫氰酸根合丁二烯的高效不对称Diels-Alder / [3,3]σ重排反应。以高收率合成了一系列环己烯基异硫氰酸酯,具有出色的非对映选择性和对映选择性。基于机理研究,提出了具有可能的过渡态模型的催化循环来解释这一过程。
更新日期:2018-06-19
中文翻译:

催化不对称Diels–Alder反应/ [1-硫氰酸根合丁二烯的[3,3]σ重排级联
通过使用手性N,N'-二氧化物/镍(II)配合物实现了亚甲基吲哚酮与1-硫氰酸根合丁二烯的高效不对称Diels-Alder / [3,3]σ重排反应。以高收率合成了一系列环己烯基异硫氰酸酯,具有出色的非对映选择性和对映选择性。基于机理研究,提出了具有可能的过渡态模型的催化循环来解释这一过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号