当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselectivity in a cyclic secondary amine catalyzed asymmetric Mannich reaction: a model rationalization from DFT studies†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1039/c8qo00424b Siwei Shu 1, 2, 3, 4, 5 , Zhao Liu 6, 7, 8, 9, 10 , Yukui Li 6, 7, 8, 9, 10 , Zhuofeng Ke 6, 7, 8, 9, 10 , Yan Liu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1039/c8qo00424b Siwei Shu 1, 2, 3, 4, 5 , Zhao Liu 6, 7, 8, 9, 10 , Yukui Li 6, 7, 8, 9, 10 , Zhuofeng Ke 6, 7, 8, 9, 10 , Yan Liu 1, 2, 3, 4, 5
Affiliation
|
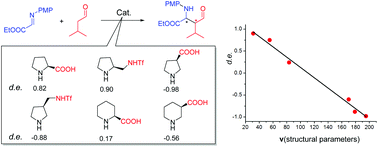
A variation in diastereoselectivity is rationalized for cyclic secondary amine catalyzed asymmetric Mannich reactions, by systematic DFT studies on a series of representative catalysis systems. Computational results well explain the experimentally observed stereoselectivities for all the systems. Structural factors that influence the stereoselectivity are elucidated based on transition state analysis. Three structural parameters including two lengths L1 and L2, and a dihedral angle D1 of intermediates are found to be able to concisely correlate the relationship between structures and diastereoselectivities. A quantitative structure-stereoselectivity relationship (QSSR) model containing these three parameters is proposed to correlate the diastereoselectivity of Mannich reactions catalyzed by cyclic secondary amines. The model rationalization study could serve as an interesting example to understand the stereoselectivities and to advance catalyst design in asymmetric organocatalysis.
中文翻译:

环状仲胺催化的不对称曼尼希反应中的非对映选择性:基于DFT研究的模型合理化†
通过对一系列代表性催化系统进行系统DFT研究,对于环仲胺催化的不对称曼尼希反应,合理化了非对映选择性的变化。计算结果很好地说明了所有系统在实验上观察到的立体选择性。基于过渡态分析,阐明了影响立体选择性的结构因素。包括两个长度L 1和L 2以及二面角D 1的三个结构参数发现中间体能够精确地关联结构与非对映选择性之间的关系。提出了包含这三个参数的定量结构-立体选择性关系(QSSR)模型,以关联环仲胺催化的曼尼希反应的非对映选择性。模型合理化研究可以作为理解立体选择性和推进不对称有机催化中催化剂设计的有趣例子。
更新日期:2018-05-24
中文翻译:

环状仲胺催化的不对称曼尼希反应中的非对映选择性:基于DFT研究的模型合理化†
通过对一系列代表性催化系统进行系统DFT研究,对于环仲胺催化的不对称曼尼希反应,合理化了非对映选择性的变化。计算结果很好地说明了所有系统在实验上观察到的立体选择性。基于过渡态分析,阐明了影响立体选择性的结构因素。包括两个长度L 1和L 2以及二面角D 1的三个结构参数发现中间体能够精确地关联结构与非对映选择性之间的关系。提出了包含这三个参数的定量结构-立体选择性关系(QSSR)模型,以关联环仲胺催化的曼尼希反应的非对映选择性。模型合理化研究可以作为理解立体选择性和推进不对称有机催化中催化剂设计的有趣例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号