当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Au-V2O5 composite nanowires through the shape transformation of a vanadium(iii) metal complex for high-performance solid-state supercapacitors†
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1039/c8qi00325d Siddheswar Rudra 1, 2, 3, 4 , Arpan Kumar Nayak 4, 5, 6, 7 , Rishika Chakraborty 1, 2, 3, 4 , Pradip K. Maji 4, 8, 9, 10 , Mukul Pradhan 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1039/c8qi00325d Siddheswar Rudra 1, 2, 3, 4 , Arpan Kumar Nayak 4, 5, 6, 7 , Rishika Chakraborty 1, 2, 3, 4 , Pradip K. Maji 4, 8, 9, 10 , Mukul Pradhan 1, 2, 3, 4
Affiliation
|
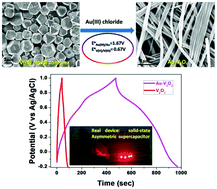
A simple redox transformation between a vanadium(III) metal complex and gold(III) chloride aided by a cost-effective modified hydrothermal procedure has been adopted for the synthesis of Au-V2O5 composite nanowires. The stability of pseudocapacitive electrode materials in acidic electrolytes is a major challenge. However, the synthesized Au-V2O5 composite nanowires are stable in acidic electrolyte when compared to the precursor component, V2O5. Electrochemical measurement shows a specific capacitance of 419 F g−1 at 1 A g−1 current density in 0.5 M H2SO4 solution for the synthesized composite nanowires. However, the precursor component V2O5 shows a lower specific capacitance under identical conditions. The synthesized composite nanowires, as a pseudocapacitive electrode material, respond to a wide range of working potential windows (+1.6 V), resulting in maximum energy and power densities of 53.33 W h kg−1 and 3.85 kW kg−1 respectively. Moreover, the Au-V2O5 nanowires show high cyclic stability (89% specific capacitance retention) for up to 5000 consecutive charge–discharge (CD) cycles at 10 A g−1 constant current density, due to the composite formation by redox transformation, which reflects the stability of the composite in acidic electrolyte.
中文翻译:

通过钒(iii)金属配合物的形状转变,合成用于高性能固态超级电容器 的Au-V 2 O 5复合纳米线†
通过成本有效的改进水热程序,在钒(III)金属配合物和氯化金(III)之间进行了简单的氧化还原转化,以合成Au-V 2 O 5复合纳米线。假电容电极材料在酸性电解质中的稳定性是一个重大挑战。然而,与前体组分V 2 O 5相比,合成的Au-V 2 O 5复合纳米线在酸性电解质中是稳定的。电化学测量显示,在0.5 MH 2 SO中,在1 A g -1电流密度下的比电容为419 F g -14解决方案,用于合成复合纳米线。但是,前体成分V 2 O 5在相同条件下显示出较低的比电容。合成的复合纳米线,作为伪电容电极材料,对宽范围的工作电势窗口(+1.6 V)做出响应,分别导致最大能量密度和功率密度分别为53.33 W h kg -1和3.85 kW kg -1。此外,Au-V 2 O 5纳米线在10 A g -1下显示了高达5000个连续的充放电(CD)循环的高循环稳定性(89%的比电容保持率)。 恒定的电流密度,这归因于通过氧化还原转化形成的复合材料,这反映了复合材料在酸性电解质中的稳定性。
更新日期:2018-05-24
中文翻译:

通过钒(iii)金属配合物的形状转变,合成用于高性能固态超级电容器 的Au-V 2 O 5复合纳米线†
通过成本有效的改进水热程序,在钒(III)金属配合物和氯化金(III)之间进行了简单的氧化还原转化,以合成Au-V 2 O 5复合纳米线。假电容电极材料在酸性电解质中的稳定性是一个重大挑战。然而,与前体组分V 2 O 5相比,合成的Au-V 2 O 5复合纳米线在酸性电解质中是稳定的。电化学测量显示,在0.5 MH 2 SO中,在1 A g -1电流密度下的比电容为419 F g -14解决方案,用于合成复合纳米线。但是,前体成分V 2 O 5在相同条件下显示出较低的比电容。合成的复合纳米线,作为伪电容电极材料,对宽范围的工作电势窗口(+1.6 V)做出响应,分别导致最大能量密度和功率密度分别为53.33 W h kg -1和3.85 kW kg -1。此外,Au-V 2 O 5纳米线在10 A g -1下显示了高达5000个连续的充放电(CD)循环的高循环稳定性(89%的比电容保持率)。 恒定的电流密度,这归因于通过氧化还原转化形成的复合材料,这反映了复合材料在酸性电解质中的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号