当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surprising formation of quasi-stable Tc(vi) in high ionic strength alkaline media†
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1039/c8qi00219c Sayandev Chatterjee 1, 2, 3, 4 , Gabriel B. Hall 1, 2, 3, 4 , Isaac E. Johnson 1, 2, 3, 4 , Yingge Du 2, 3, 4, 5 , Eric D. Walter 2, 3, 4, 6 , Nancy M. Washton 2, 3, 4, 6 , Tatiana G. Levitskaia 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2018-05-24 00:00:00 , DOI: 10.1039/c8qi00219c Sayandev Chatterjee 1, 2, 3, 4 , Gabriel B. Hall 1, 2, 3, 4 , Isaac E. Johnson 1, 2, 3, 4 , Yingge Du 2, 3, 4, 5 , Eric D. Walter 2, 3, 4, 6 , Nancy M. Washton 2, 3, 4, 6 , Tatiana G. Levitskaia 1, 2, 3, 4
Affiliation
|
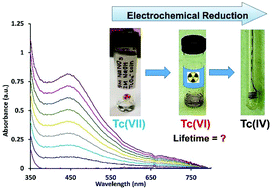
Despite decades of research, the reduction behavior of pertechnetate, TcO4−, is not well understood and represents one of the largest knowledge gaps in the transition metal series. Conventional wisdom presumes the reduction of TcO4− in aqueous systems to predominantly follow either a rapid two electron, or three electron process to form either a TcV or TcIV species. This study for the first time demonstrates the importance of the TcVI species through systematic characterization by multiple spectroscopic techniques. The reduction of TcO4− was examined in a matrix of 5 M NaNO3 at 0–2 M NaOH. Results of the cyclic voltammetric evaluation of reduction to TcVI, Nernstian analysis of the visible spectroelectrochemical signal, and structural spectroscopic EPR and XPS analysis of the reduction product generated by bulk electrolysis consistently support an electron transfer stoichiometry corresponding to a 1e− reduction. This TcVI species is markedly more stable than had been previously considered, with decomposition kinetics that correspond to a half-life of 1.91 ± 0.07 days, fully 6 orders of magnitude longer than previously reported for an aqueous solution. These results reveal the importance of a TcVI intermediate species in high ionic strength alkaline solutions and significantly contribute to the understanding of the mechanism of TcO4− reduction and formation of low-valent Tc species.
中文翻译:

在高离子强度碱性介质中 惊人地形成准稳定的Tc(vi)†
尽管数十年的研究,高锝酸盐的减持行为,TCO 4 - ,还不是很清楚,并表示在过渡金属系列最大的知识差距之一。传统智慧假定TCO的还原4 -在水性体系中,以主要遵循任一快速两个电子,或三个电子处理,以形成一个任锝V或TC IV物种。这项研究首次通过多种光谱技术对Tc VI物种进行了系统表征,证明了其重要性。TCO的还原4 -中的5M的NaNO的基质检查3在0–2 M NaOH中。减少到锝的循环伏安评价结果VI,可见光谱电化学信号的能斯特分析和结构分光EPR和通过本体电解所产生一贯支持对应于一个1E电子转移化学计量的还原产物的XPS分析-还原。这种Tc VI物质比以前认为的要稳定得多,其分解动力学相当于1.91±0.07天的半衰期,比以前报道的水溶液要长6个数量级。这些结果揭示了Tc VI的重要性在高离子强度的碱性溶液中间物种和显著有助于TCO的机制的理解4 -还原和形成低原子价锝物种。
更新日期:2018-05-24
中文翻译:

在高离子强度碱性介质中 惊人地形成准稳定的Tc(vi)†
尽管数十年的研究,高锝酸盐的减持行为,TCO 4 - ,还不是很清楚,并表示在过渡金属系列最大的知识差距之一。传统智慧假定TCO的还原4 -在水性体系中,以主要遵循任一快速两个电子,或三个电子处理,以形成一个任锝V或TC IV物种。这项研究首次通过多种光谱技术对Tc VI物种进行了系统表征,证明了其重要性。TCO的还原4 -中的5M的NaNO的基质检查3在0–2 M NaOH中。减少到锝的循环伏安评价结果VI,可见光谱电化学信号的能斯特分析和结构分光EPR和通过本体电解所产生一贯支持对应于一个1E电子转移化学计量的还原产物的XPS分析-还原。这种Tc VI物质比以前认为的要稳定得多,其分解动力学相当于1.91±0.07天的半衰期,比以前报道的水溶液要长6个数量级。这些结果揭示了Tc VI的重要性在高离子强度的碱性溶液中间物种和显著有助于TCO的机制的理解4 -还原和形成低原子价锝物种。



























 京公网安备 11010802027423号
京公网安备 11010802027423号