当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt(III)‐Catalyzed [4+2] Annulation of Heterobicyclic Alkenes by sp2 C−H Activation
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-13 , DOI: 10.1002/ajoc.201800251 Arnab Dey 1 , Anuja Rathi 1 , Chandra M. R. Volla 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-13 , DOI: 10.1002/ajoc.201800251 Arnab Dey 1 , Anuja Rathi 1 , Chandra M. R. Volla 1
Affiliation
|
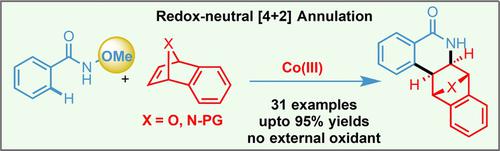
An efficient and convenient cobalt(III)‐catalyzed highly diastereoselective strategy was demonstrated for the synthesis of epoxybenzophenanthridinone derivatives. The sp2 C−H activation of N‐methoxybenzamides and their annulation reaction with 7‐oxa/aza benzonorbornadienes proceeds under mild conditions and exhibits excellent functional group tolerance. In addition, the products were transformed to biologically relevant phenanthridinones by an acid‐catalyzed ring opening/aromatization sequence.
中文翻译:

钴(III)催化sp2 CH活化的杂环双烯环[4 + 2]
证明了一种有效且方便的钴(III)催化的高度非对映选择性策略可用于合成环氧苯并菲蒽酮衍生物。N-甲氧基苯甲酰胺的sp 2 C H活化及其与7-氧杂/氮杂苯并降冰片二烯的环化反应在温和的条件下进行,并表现出出色的官能团耐受性。此外,通过酸催化的开环/芳构化序列,将产物转化为生物学上相关的菲啶酮。
更新日期:2018-06-13
中文翻译:

钴(III)催化sp2 CH活化的杂环双烯环[4 + 2]
证明了一种有效且方便的钴(III)催化的高度非对映选择性策略可用于合成环氧苯并菲蒽酮衍生物。N-甲氧基苯甲酰胺的sp 2 C H活化及其与7-氧杂/氮杂苯并降冰片二烯的环化反应在温和的条件下进行,并表现出出色的官能团耐受性。此外,通过酸催化的开环/芳构化序列,将产物转化为生物学上相关的菲啶酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号