Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activity-dependent neuroprotective protein recruits HP1 and CHD4 to control lineage-specifying genes
Nature ( IF 50.5 ) Pub Date : 2018-05-01 , DOI: 10.1038/s41586-018-0153-8 Veronika Ostapcuk , Fabio Mohn , Sarah H. Carl , Anja Basters , Daniel Hess , Vytautas Iesmantavicius , Lisa Lampersberger , Matyas Flemr , Aparna Pandey , Nicolas H. Thomä , Joerg Betschinger , Marc Bühler
Nature ( IF 50.5 ) Pub Date : 2018-05-01 , DOI: 10.1038/s41586-018-0153-8 Veronika Ostapcuk , Fabio Mohn , Sarah H. Carl , Anja Basters , Daniel Hess , Vytautas Iesmantavicius , Lisa Lampersberger , Matyas Flemr , Aparna Pandey , Nicolas H. Thomä , Joerg Betschinger , Marc Bühler
|
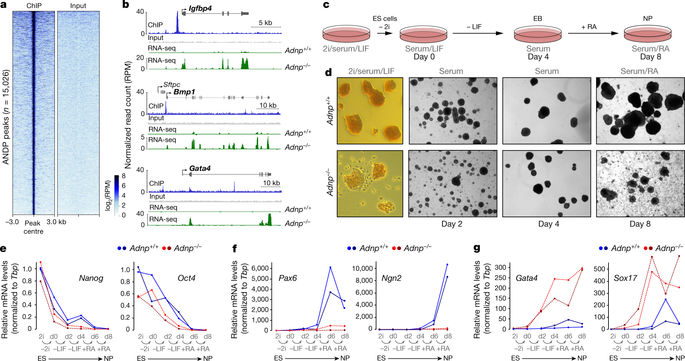
De novo mutations in ADNP, which encodes activity-dependent neuroprotective protein (ADNP), have recently been found to underlie Helsmoortel–Van der Aa syndrome, a complex neurological developmental disorder that also affects several other organ functions1. ADNP is a putative transcription factor that is essential for embryonic development2. However, its precise roles in transcriptional regulation and development are not understood. Here we show that ADNP interacts with the chromatin remodeller CHD4 and the chromatin architectural protein HP1 to form a stable complex, which we refer to as ChAHP. Besides mediating complex assembly, ADNP recognizes DNA motifs that specify binding of ChAHP to euchromatin. Genetic ablation of ChAHP components in mouse embryonic stem cells results in spontaneous differentiation concomitant with premature activation of lineage-specific genes and in a failure to differentiate towards the neuronal lineage. Molecularly, ChAHP-mediated repression is fundamentally different from canonical HP1-mediated silencing: HP1 proteins, in conjunction with histone H3 lysine 9 trimethylation (H3K9me3), are thought to assemble broad heterochromatin domains that are refractory to transcription. ChAHP-mediated repression, however, acts in a locally restricted manner by establishing inaccessible chromatin around its DNA-binding sites and does not depend on H3K9me3-modified nucleosomes. Together, our results reveal that ADNP, via the recruitment of HP1 and CHD4, regulates the expression of genes that are crucial for maintaining distinct cellular states and assures accurate cell fate decisions upon external cues. Such a general role of ChAHP in governing cell fate plasticity may explain why ADNP mutations affect several organs and body functions and contribute to cancer progression1,3,4. Notably, we found that the integrity of the ChAHP complex is disrupted by nonsense mutations identified in patients with Helsmoortel–Van der Aa syndrome, and this could be rescued by aminoglycosides that suppress translation termination5. Therefore, patients might benefit from therapeutic agents that are being developed to promote ribosomal read-through of premature stop codons6,7.ADNP interacts with the chromatin remodeller CHD4 and the heterochromatin protein HP1 to form a complex termed ChAHP that represses gene expression independently of the histone H3K9me3 modification.
中文翻译:

活动依赖性神经保护蛋白招募 HP1 和 CHD4 来控制谱系特异性基因
最近发现 ADNP 中的从头突变是 Helsmoortel-Van der Aa 综合征的基础,它编码活动依赖性神经保护蛋白 (ADNP),这是一种复杂的神经发育障碍,也会影响其他几种器官功能。ADNP 是一种假定的转录因子,对胚胎发育至关重要。然而,它在转录调控和发育中的确切作用尚不清楚。在这里,我们展示了 ADNP 与染色质重塑剂 CHD4 和染色质结构蛋白 HP1 相互作用以形成稳定的复合物,我们将其称为 ChAHP。除了介导复杂的组装外,ADNP 还识别指定 ChAHP 与常染色质结合的 DNA 基序。小鼠胚胎干细胞中 ChAHP 成分的遗传消融导致自发分化,伴随着谱系特异性基因的过早激活,并且无法向神经元谱系分化。在分子上,ChAHP 介导的抑制与经典的 HP1 介导的沉默有着根本的不同:HP1 蛋白与组蛋白 H3 赖氨酸 9 三甲基化 (H3K9me3) 一起被认为组装了难以转录的广泛异染色质域。然而,ChAHP 介导的抑制通过在其 DNA 结合位点周围建立难以接近的染色质以局部受限的方式起作用,并且不依赖于 H3K9me3 修饰的核小体。总之,我们的结果表明 ADNP 通过招募 HP1 和 CHD4,调节对维持不同细胞状态至关重要的基因的表达,并确保根据外部线索做出准确的细胞命运决定。ChAHP 在控制细胞命运可塑性方面的这种普遍作用可以解释为什么 ADNP 突变会影响多个器官和身体功能并导致癌症进展 1,3,4。值得注意的是,我们发现在 Helsmoortel-Van der Aa 综合征患者中发现的无义突变破坏了 ChAHP 复合物的完整性,这可以通过抑制翻译终止的氨基糖苷类药物来挽救。因此,患者可能会受益于正在开发的治疗药物,以促进过早终止密码子的核糖体通读 6,7。
更新日期:2018-05-01
中文翻译:

活动依赖性神经保护蛋白招募 HP1 和 CHD4 来控制谱系特异性基因
最近发现 ADNP 中的从头突变是 Helsmoortel-Van der Aa 综合征的基础,它编码活动依赖性神经保护蛋白 (ADNP),这是一种复杂的神经发育障碍,也会影响其他几种器官功能。ADNP 是一种假定的转录因子,对胚胎发育至关重要。然而,它在转录调控和发育中的确切作用尚不清楚。在这里,我们展示了 ADNP 与染色质重塑剂 CHD4 和染色质结构蛋白 HP1 相互作用以形成稳定的复合物,我们将其称为 ChAHP。除了介导复杂的组装外,ADNP 还识别指定 ChAHP 与常染色质结合的 DNA 基序。小鼠胚胎干细胞中 ChAHP 成分的遗传消融导致自发分化,伴随着谱系特异性基因的过早激活,并且无法向神经元谱系分化。在分子上,ChAHP 介导的抑制与经典的 HP1 介导的沉默有着根本的不同:HP1 蛋白与组蛋白 H3 赖氨酸 9 三甲基化 (H3K9me3) 一起被认为组装了难以转录的广泛异染色质域。然而,ChAHP 介导的抑制通过在其 DNA 结合位点周围建立难以接近的染色质以局部受限的方式起作用,并且不依赖于 H3K9me3 修饰的核小体。总之,我们的结果表明 ADNP 通过招募 HP1 和 CHD4,调节对维持不同细胞状态至关重要的基因的表达,并确保根据外部线索做出准确的细胞命运决定。ChAHP 在控制细胞命运可塑性方面的这种普遍作用可以解释为什么 ADNP 突变会影响多个器官和身体功能并导致癌症进展 1,3,4。值得注意的是,我们发现在 Helsmoortel-Van der Aa 综合征患者中发现的无义突变破坏了 ChAHP 复合物的完整性,这可以通过抑制翻译终止的氨基糖苷类药物来挽救。因此,患者可能会受益于正在开发的治疗药物,以促进过早终止密码子的核糖体通读 6,7。











































 京公网安备 11010802027423号
京公网安备 11010802027423号