当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Tricopper(I) Complex Competent for O Atom Transfer, C–H Bond Activation, and Multiple O2 Activation Steps
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00921 Brian J. Cook 1 , Gianna N. Di Francesco 1 , Matthew T. Kieber-Emmons 2 , Leslie J. Murray 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00921 Brian J. Cook 1 , Gianna N. Di Francesco 1 , Matthew T. Kieber-Emmons 2 , Leslie J. Murray 1
Affiliation
|
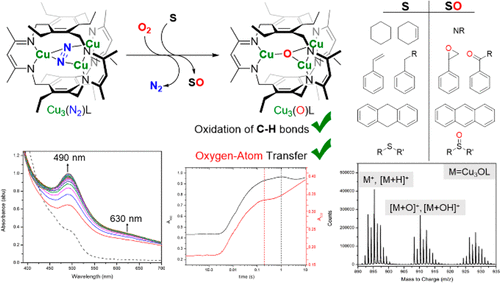
Oxygenation of a tricopper(I) cyclophanate (1) affords reactive transients competent for C–H bond activation and O atom transfer to various substrates (including toluene, dihydroanthracene, and ethylmethylsulfide) based on 1H NMR, gas chromatography/mass spectrometry (MS), and electrospray ionization (ESI)/MS data. Low product yields (<1%) are determined for C–H activation substrates (e.g, toluene, ethylbenzene), which we attribute to competitive ligand oxidation. The combined stopped-flow UV/visible, electron paramagnetic resonance, ESI/MS, 1H NMR, and density functional theory (DFT) results for reaction of 1 with O2 are consistent with transient peroxo- and di(oxo)-bridged intermediates. DFT calculations elucidate a concerted proton-coupled electron transfer from toluene to the di(μ-oxo) intermediate and subsequent radical rebound as the C–H activation mechanism. Our results support a multicopper oxidase-like mechanism for O2 activation by 1, traversing species similar to the coplanar Cu3O2 unit in the peroxy and native intermediates.
中文翻译:

具有O原子转移,CH键活化和多个O 2活化步骤的Tricopper(I)复合物
一个tricopper的氧合(I)cyclophanate(1)得到的反应性瞬变胜任C-H键活化和O原子转移到各种基材(包括甲苯,二氢蒽,和ethylmethylsulfide)基于1 H NMR,气相色谱/质谱(MS )和电喷雾电离(ESI)/ MS数据。确定了C–H活化底物(例如甲苯,乙苯)的低产品收率(<1%),这归因于竞争性配体氧化。1与O 2反应的合成停止流UV /可见光,电子顺磁共振,ESI / MS,1 H NMR和密度泛函理论(DFT)结果与瞬时过氧桥和二氧桥桥接的中间体一致。DFT计算阐明了质子耦合电子从甲苯向二(μ-氧代)中间体的协调转移以及随后的作为CH活化机理的自由基反弹。我们的研究结果支持了一种通过1激活O 2的多铜氧化酶样机制,遍历过氧化物和天然中间体中与共面Cu 3 O 2单元相似的物种。
更新日期:2018-05-23
中文翻译:

具有O原子转移,CH键活化和多个O 2活化步骤的Tricopper(I)复合物
一个tricopper的氧合(I)cyclophanate(1)得到的反应性瞬变胜任C-H键活化和O原子转移到各种基材(包括甲苯,二氢蒽,和ethylmethylsulfide)基于1 H NMR,气相色谱/质谱(MS )和电喷雾电离(ESI)/ MS数据。确定了C–H活化底物(例如甲苯,乙苯)的低产品收率(<1%),这归因于竞争性配体氧化。1与O 2反应的合成停止流UV /可见光,电子顺磁共振,ESI / MS,1 H NMR和密度泛函理论(DFT)结果与瞬时过氧桥和二氧桥桥接的中间体一致。DFT计算阐明了质子耦合电子从甲苯向二(μ-氧代)中间体的协调转移以及随后的作为CH活化机理的自由基反弹。我们的研究结果支持了一种通过1激活O 2的多铜氧化酶样机制,遍历过氧化物和天然中间体中与共面Cu 3 O 2单元相似的物种。



























 京公网安备 11010802027423号
京公网安备 11010802027423号