当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption Thermodynamics and Kinetics of Light Hydrocarbons on Microporous Activated Carbon at Low Temperatures
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-06-05 , DOI: 10.1021/acs.iecr.8b00678 Florian Birkmann 1 , Christoph Pasel 1 , Michael Luckas 1 , Dieter Bathen 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-06-05 , DOI: 10.1021/acs.iecr.8b00678 Florian Birkmann 1 , Christoph Pasel 1 , Michael Luckas 1 , Dieter Bathen 1, 2
Affiliation
|
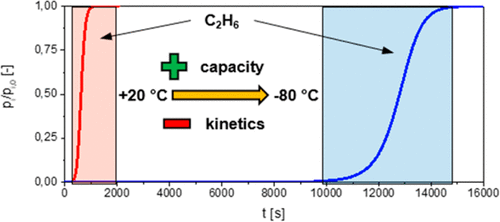
The removal of light hydrocarbons from exhaust air and process gas is important for a variety of applications, e.g., in natural-gas treatment. However, particularly at lower concentrations, the removal of C1 and C2 hydrocarbons is either very expensive or unfeasible with standard technology. Adsorption processes at temperatures below 0 °C may provide a technical solution, but until today, no systematic study on the dynamics of trace adsorption at low temperatures is available. Therefore, in this work, we present breakthrough curve experiments of ethane, propane, and n-butane over a temperature range from +20 to −80 °C and a concentration range from 5 to 1000 Pa on microporous activated carbon. Equilibrium loadings are calculated and modeled with the temperature-dependent Toth equation. From dynamic simulations of the experimental breakthrough curves, kinetic parameters are determined. The lowering of temperature results in the slowdown of kinetics, which, however, is overcompensated by a simultaneous capacity gain.
中文翻译:

低温下轻烃在微孔活性炭上的吸附热力学和动力学
从废气和工艺气体中去除轻烃对于多种应用(例如,天然气处理)很重要。但是,特别是在较低浓度下,用标准技术除去C 1和C 2烃非常昂贵或不可行。低于0°C的温度下的吸附过程可能会提供一种技术解决方案,但是直到今天,仍无法对低温下痕量吸附的动力学进行系统的研究。因此,在这项工作中,我们乙烷的存在穿透曲线实验,丙烷和ñ-丁烷在+20至-80°C的温度范围内以及在微孔活性炭上的浓度范围为5至1000 Pa。使用与温度相关的Toth方程来计算平衡负载并对其进行建模。从实验突破曲线的动态模拟,确定动力学参数。温度降低导致动力学减慢,但是,同时进行的容量增加会对其过度补偿。
更新日期:2018-06-05
中文翻译:

低温下轻烃在微孔活性炭上的吸附热力学和动力学
从废气和工艺气体中去除轻烃对于多种应用(例如,天然气处理)很重要。但是,特别是在较低浓度下,用标准技术除去C 1和C 2烃非常昂贵或不可行。低于0°C的温度下的吸附过程可能会提供一种技术解决方案,但是直到今天,仍无法对低温下痕量吸附的动力学进行系统的研究。因此,在这项工作中,我们乙烷的存在穿透曲线实验,丙烷和ñ-丁烷在+20至-80°C的温度范围内以及在微孔活性炭上的浓度范围为5至1000 Pa。使用与温度相关的Toth方程来计算平衡负载并对其进行建模。从实验突破曲线的动态模拟,确定动力学参数。温度降低导致动力学减慢,但是,同时进行的容量增加会对其过度补偿。











































 京公网安备 11010802027423号
京公网安备 11010802027423号