当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Physical Characterization of Frozen Saltwater Solutions Using Raman Microscopy
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00045 Philip P. A. Malley 1 , Subha Chakraborty 1 , Tara F. Kahan 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00045 Philip P. A. Malley 1 , Subha Chakraborty 1 , Tara F. Kahan 1
Affiliation
|
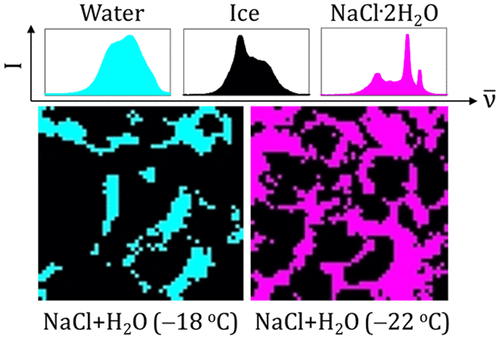
Ice is an important but poorly understood atmospheric reaction medium. Reactions in ice and at air–ice interfaces are often modeled using rate constants measured in liquid aqueous solution, despite evidence that reactivity in these two media can be very different. This approach may be valid at high ionic strengths (e.g., in sea ice) as a result of the formation of liquid brine. However, recent experiments indicate uneven solute distribution at ice surfaces, suggesting that liquid water does not completely wet ice surfaces at environmentally relevant solute concentrations. We have investigated the distribution of liquid solution, solid ice, and solid salt (NaCl·2H2O, “hydrohalite”) at the surface of frozen aqueous sodium chloride (NaCl) solutions and frozen seawater using Raman microscopy. At temperatures above the eutectic temperature (−21.1 °C), the ice surfaces were incompletely wetted, except occasionally at the highest temperatures (approximately −5 °C). Liquid water at the surface took the form of either isolated patches or channels, depending upon the salt concentration and sample temperature; liquid fractions ranged from approximately 11 to 85%. Three-dimensional (“volume”) maps showed similar liquid fractions and channel widths at all depths investigated (up to 100 μm) as well as at the surface for each sample composition. Below −21.1 °C, no liquid was observed in any sample. Instead, hydrohalite was observed with surface coverages ranging from 13 to 100% depending upon the salt concentration; surface coverage was independent of temperature between −30 and −22 °C. Accounting for the presence of two distinct reaction environments at the surface of salty ice might improve predictions of physical and chemical processes in snow-covered regions.
中文翻译:

使用拉曼显微镜对冷冻咸水溶液进行物理表征
冰是一种重要的但知之甚少的大气反应介质。尽管有证据表明在这两种介质中的反应性可能大不相同,但通常使用在液态水溶液中测得的速率常数来模拟冰和气冰界面中的反应。由于形成液体盐水,该方法在高离子强度下(例如在海冰中)可能是有效的。但是,最近的实验表明,溶质在冰面上的分布不均匀,表明液态水在与环境相关的溶质浓度下不能完全润湿冰面。我们研究了液体溶液,固体冰和固体盐(NaCl·2H 2O,使用拉曼显微镜在冷冻的氯化钠水溶液(NaCl)和冷冻的海水表面上的“卤化氢”。在高于共晶温度(−21.1°C)的温度下,除偶尔在最高温度(约-5°C)外,冰面未完全润湿。根据盐浓度和样品温度的不同,表面的液态水呈孤立斑块或通道的形式。液体馏分的范围从大约11%到85%。三维(“体积”)图在每种样品成分的所有调查深度(最大100μm)以及表面均显示相似的液体馏分和通道宽度。低于-21.1°C,在任何样品中均未观察到液体。取而代之的是,观察到水卤石的表面覆盖率取决于盐浓度为13%至100%;表面覆盖范围与−30至−22°C之间的温度无关。考虑到咸冰表面存在两种不同的反应环境,可能会改善对冰雪覆盖地区的物理和化学过程的预测。
更新日期:2018-05-23
中文翻译:

使用拉曼显微镜对冷冻咸水溶液进行物理表征
冰是一种重要的但知之甚少的大气反应介质。尽管有证据表明在这两种介质中的反应性可能大不相同,但通常使用在液态水溶液中测得的速率常数来模拟冰和气冰界面中的反应。由于形成液体盐水,该方法在高离子强度下(例如在海冰中)可能是有效的。但是,最近的实验表明,溶质在冰面上的分布不均匀,表明液态水在与环境相关的溶质浓度下不能完全润湿冰面。我们研究了液体溶液,固体冰和固体盐(NaCl·2H 2O,使用拉曼显微镜在冷冻的氯化钠水溶液(NaCl)和冷冻的海水表面上的“卤化氢”。在高于共晶温度(−21.1°C)的温度下,除偶尔在最高温度(约-5°C)外,冰面未完全润湿。根据盐浓度和样品温度的不同,表面的液态水呈孤立斑块或通道的形式。液体馏分的范围从大约11%到85%。三维(“体积”)图在每种样品成分的所有调查深度(最大100μm)以及表面均显示相似的液体馏分和通道宽度。低于-21.1°C,在任何样品中均未观察到液体。取而代之的是,观察到水卤石的表面覆盖率取决于盐浓度为13%至100%;表面覆盖范围与−30至−22°C之间的温度无关。考虑到咸冰表面存在两种不同的反应环境,可能会改善对冰雪覆盖地区的物理和化学过程的预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号