European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-05-23 , DOI: 10.1016/j.ejmech.2018.05.031 Roshanak Ghobadian , Mohammad Mahdavi , Hamid Nadri , Alireza Moradi , Najmeh Edraki , Tahmineh Akbarzadeh , Mohammad Sharifzadeh , Syed Nasir Abbas Bukhari , Mohsen Amini
|
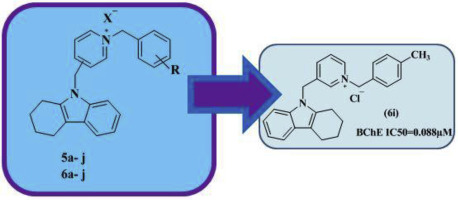
Butyrylcholinesterase (BuChE) inhibitors have become interesting target for treatment of Alzheimer's disease (AD). A series of dual binding site BuChE inhibitors were designed and synthesized based on 2,3,4,9-tetrahydro-1H-carbazole attached benzyl pyridine moieties. In-vitro assay revealed that all of the designed compounds were selective and potent BuChE inhibitors. The most potent BuChE inhibitor was compound 6i (IC50 = 0.088 ± 0.0009 μM) with the mixed-type inhibition. Docking study revealed that 6i is a dual binding site BuChE inhibitor. Also, Pharmacokinetic properties for 6i were accurate to Lipinski's rule. In addition, compound 6i demonstrated neuroprotective and β-secretase (BACE1) inhibition activities. This compound could also inhibit AChE-induced and self-induced Aβ peptide aggregation at concentration of 100 μM and 10 μM respectively. Generally, the results are presented as new potent selective BuChE inhibitors with a therapeutic potential for the treatment of AD.
中文翻译:

新型四氢咔唑苄基吡啶杂化物作为有效的和选择性的丁酰胆碱酯酶抑制剂,具有神经保护和β-分泌酶抑制活性
丁酰胆碱酯酶(BuChE)抑制剂已成为治疗阿尔茨海默氏病(AD)的有趣靶标。基于2,3,4,9-四氢-1H-咔唑连接的苄基吡啶部分,设计和合成了一系列双结合位点BuChE抑制剂。体外测定表明,所有设计的化合物均为选择性和有效的BuChE抑制剂。最有效的BuChE抑制剂是化合物6i(IC 50 = 0.088±0.0009μM),具有混合型抑制作用。对接研究表明6i是BuChE双重结合位点抑制剂。同样,6i的药代动力学特性与Lipinski的定律是准确的。此外,化合物6i表现出神经保护和β-分泌酶(BACE1)抑制活性。该化合物还可以分别在100μM和10μM的浓度下抑制AChE诱导的Aβ肽和自身诱导的Aβ肽聚集。通常,结果显示为具有治疗AD潜在治疗潜力的新型有效BuChE抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号