Tetrahedron ( IF 2.1 ) Pub Date : 2018-05-23 , DOI: 10.1016/j.tet.2018.05.065 Sankar Mohan , John R. Thompson , B. Mario Pinto , Andrew J. Bennet
|
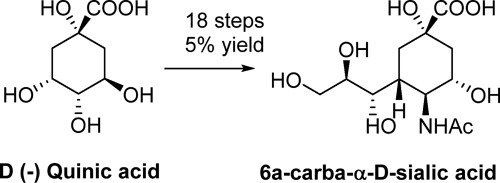
Sialic acid (N-acetylneuraminic acid) is a carbohydrate that possess a nine carbon backbone, and it is often found at the termini of glycoconjugates in biological systems. Because of this prominence many syntheses have reported routes to sialic acid and many of its derivatives. Most of these compounds retain the endocyclic oxygen atom that becomes part of the ketal glycosidic linkage that joins sialic acid to the penultimate residue in the glycoconjugate. With respect to carba-sialic acid (replacement of the ring oxygen atom with a methylene group) a single synthesis has been reported (Ogawa et al. (Carbohydr. Res., 1995, 269, 53–78) in 30 steps and 0.5% yield. The current report details a robust synthesis of 6a-carba-α-d-sialic acid that involves 18 steps and give a 5% yield using d-quinic acid as the starting material.
中文翻译:

多功能合成碳环N-乙酰神经氨酸及其衍生物的途径
唾液酸(N-乙酰神经氨酸)是一种具有9个碳主链的碳水化合物,通常在生物系统中的糖缀合物末端被发现。由于这一突出,已报道了许多合成唾液酸及其许多衍生物的途径。这些化合物中的大多数保留了环内氧原子,后者成为缩酮糖苷键的一部分,缩酮糖苷键将唾液酸连接到糖缀合物中的倒数第二个残基上。相对于碳代唾液酸(与亚甲基组替换所述环氧原子)的单个合成已经报道(Ogawa等人(Carbohydr。RES。 ,1995年,269在30步和0.5%,53-78)本报告详细介绍了6a-carba-α-的可靠合成方法d-唾液酸涉及18个步骤,并以d-奎尼酸为起始原料可得到5%的收率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号