当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
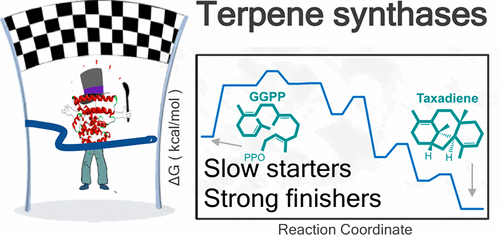
Slow-Starter Enzymes: Role of Active-Site Architecture in the Catalytic Control of the Biosynthesis of Taxadiene by Taxadiene Synthase
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.biochem.8b00452 Tamar Ansbacher 1, 2 , Yehoshua Freud 1 , Dan Thomas Major 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.biochem.8b00452 Tamar Ansbacher 1, 2 , Yehoshua Freud 1 , Dan Thomas Major 1
Affiliation
|
Taxadiene synthase (TXS) catalyzes the formation of natural product taxa-4(5),11(12)-diene (henceforth taxadiene). Taxadiene is the precursor in the formation of Taxol, which is an important natural anticancer agent. In the current study, we present a detailed mechanistic view of the biosynthesis of taxadiene by TXS using a hybrid quantum mechanics-molecular mechanics potential in conjunction with free energy simulation methods. The obtained free-energy landscape displays initial endergonic steps followed by a stepwise downhill profile, which is an emerging free-energy fingerprint for type I terpene synthases. We identify an active-site Trp residue (W753) as a key feature of the TXS active-site architecture and propose that this residue stabilized intermediate cations via π-cation interactions. To validate our proposed active TXS model, we examine a previously reported W753H mutation, which leads to the exclusive formation of side product cembrene A. The simulations of the W753H mutant show that, in the mutant structure, the His side chain is in the perfect position to deprotonate the cembrenyl cation en route to cembrene formation and that this abortive deprotonation is an energetically facile process. On the basis of the current model, we propose that an analogous mutation of Y841 to His could possibly lead to verticillane. The current simulations stress the importance of the precise positioning of key active-site residues in stabilizing intermediate carbocations. In view of the great pharmaceutical importance of taxadiene, a detailed understanding of the TXS mechanism can provide important clues toward a synthetic strategy for Taxol manufacturing.
中文翻译:

慢启动酶:活性站点体系结构在紫杉二烯合酶催化控制紫杉二烯生物合成中的作用
紫杉二烯合酶(TXS)催化天然产物紫杉类4(5),11(12)-二烯(此后称为紫杉二烯)的形成。紫杉二烯是紫杉醇形成的前体,紫杉醇是重要的天然抗癌剂。在当前的研究中,我们提出了使用混合量子力学-分子力学势结合自由能模拟方法通过TXS进行紫杉二烯生物合成的详细机理视图。所获得的自由能景观显示出初始的阶跃阶跃,然后是逐步下坡的轮廓,这是I型萜烯合酶的新兴自由能指纹图谱。我们将一个活性位点Trp残基(W753)确定为TXS活性位点体系结构的关键特征,并提出该残基通过π-阳离子相互作用稳定了中间阳离子。为了验证我们提出的主动TXS模型,我们检查了先前报道的W753H突变,该突变导致副产物西柏烯A的排他性形成。W753H突变体的模拟显示,在突变体结构中,His侧链处于理想的位置,可以在途中使西松烯阳离子去质子化到西伯仑形成,并且这种流产的去质子化是一个能量上容易的过程。在当前模型的基础上,我们建议将Y841与His进行类似的突变可能导致黄萎病。当前的模拟强调了关键活性位点残基的精确定位在稳定中间碳正离子中的重要性。考虑到紫杉二烯的巨大药学重要性,对TXS机理的详细了解可以为紫杉醇生产的合成策略提供重要线索。
更新日期:2018-05-23
中文翻译:

慢启动酶:活性站点体系结构在紫杉二烯合酶催化控制紫杉二烯生物合成中的作用
紫杉二烯合酶(TXS)催化天然产物紫杉类4(5),11(12)-二烯(此后称为紫杉二烯)的形成。紫杉二烯是紫杉醇形成的前体,紫杉醇是重要的天然抗癌剂。在当前的研究中,我们提出了使用混合量子力学-分子力学势结合自由能模拟方法通过TXS进行紫杉二烯生物合成的详细机理视图。所获得的自由能景观显示出初始的阶跃阶跃,然后是逐步下坡的轮廓,这是I型萜烯合酶的新兴自由能指纹图谱。我们将一个活性位点Trp残基(W753)确定为TXS活性位点体系结构的关键特征,并提出该残基通过π-阳离子相互作用稳定了中间阳离子。为了验证我们提出的主动TXS模型,我们检查了先前报道的W753H突变,该突变导致副产物西柏烯A的排他性形成。W753H突变体的模拟显示,在突变体结构中,His侧链处于理想的位置,可以在途中使西松烯阳离子去质子化到西伯仑形成,并且这种流产的去质子化是一个能量上容易的过程。在当前模型的基础上,我们建议将Y841与His进行类似的突变可能导致黄萎病。当前的模拟强调了关键活性位点残基的精确定位在稳定中间碳正离子中的重要性。考虑到紫杉二烯的巨大药学重要性,对TXS机理的详细了解可以为紫杉醇生产的合成策略提供重要线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号