当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lewis‐Base‐Catalyzed Domino Reaction of Morita–Baylis–Hillman Carbonates of Isatins with Enolizable Cyclic Carbonyl Compounds: Stereoselective Access to Spirooxindole‐Pyrans
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-06-22 , DOI: 10.1002/ajoc.201800240 Anubha Yadav 1 , Joyanta Banerjee 1 , Sanjeeva K. Arupula 1 , Shaikh M. Mobin 1 , Sampak Samanta 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-06-22 , DOI: 10.1002/ajoc.201800240 Anubha Yadav 1 , Joyanta Banerjee 1 , Sanjeeva K. Arupula 1 , Shaikh M. Mobin 1 , Sampak Samanta 1
Affiliation
|
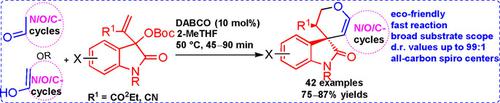
The present paper reports an efficient, organocatalytic, environmentally friendly and stereoselective [3+3] one‐pot allylic alkylation/oxa‐Michael reaction by involving a wide range of Morita–Baylis–Hillman (MBH) carbonates of isatins and several pharmacologically attractive enolizable C−H activated cyclic carbonyl compounds such as 1,3‐binucleophiles, namely pyrazolones, isoxazolones, 4‐hydroxyxoumarins, 4‐hydroxy‐6‐methyl‐α‐pyrone, lawsone and dimedone in a biomass‐derived 2‐MeTHF as green solvent catalyzed by DABCO as a solid Lewis‐base catalyst. This protocol delivers a unique class of medicinally promising spirooxindole‐fused‐dihydropyran scaffolds.
中文翻译:

Isitas的Morita–Baylis–Hillman碳酸盐与可烯丙基环状羰基化合物的Lewis碱催化多米诺反应:立体选择进入螺氧杂吲哚-吡喃
本论文报告了一种有效的,有机催化的,对环境友好的和立体选择性的[3 + 3]单锅烯丙基烷基化/ oxa-Michael反应,其中涉及广泛的靛红Morita-Baylis-Hillman(MBH)碳酸盐以及几种具有药理吸引力的烯醇化由C-H活化的环状羰基化合物,例如1,3-双亲核试剂,即吡唑啉酮,异恶唑啉酮,4-羟基黄豆香素,4-羟基-6-甲基-α-吡喃酮,Lawone和二甲基酮在绿色生物质衍生的2-MeTHF中作为绿色溶剂DABCO催化的固体路易斯碱催化剂。该方案提供了一类独特的具有医学前景的螺环吲哚融合的二氢吡喃支架。
更新日期:2018-06-22
中文翻译:

Isitas的Morita–Baylis–Hillman碳酸盐与可烯丙基环状羰基化合物的Lewis碱催化多米诺反应:立体选择进入螺氧杂吲哚-吡喃
本论文报告了一种有效的,有机催化的,对环境友好的和立体选择性的[3 + 3]单锅烯丙基烷基化/ oxa-Michael反应,其中涉及广泛的靛红Morita-Baylis-Hillman(MBH)碳酸盐以及几种具有药理吸引力的烯醇化由C-H活化的环状羰基化合物,例如1,3-双亲核试剂,即吡唑啉酮,异恶唑啉酮,4-羟基黄豆香素,4-羟基-6-甲基-α-吡喃酮,Lawone和二甲基酮在绿色生物质衍生的2-MeTHF中作为绿色溶剂DABCO催化的固体路易斯碱催化剂。该方案提供了一类独特的具有医学前景的螺环吲哚融合的二氢吡喃支架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号