当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silylarene Hydrogenation: A Strategic Approach that Enables Direct Access to Versatile Silylated Saturated Carbo‐ and Heterocycles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-21 , DOI: 10.1002/anie.201804124 Mario P. Wiesenfeldt 1 , Tobias Knecht 1 , Christoph Schlepphorst 1 , Frank Glorius 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-21 , DOI: 10.1002/anie.201804124 Mario P. Wiesenfeldt 1 , Tobias Knecht 1 , Christoph Schlepphorst 1 , Frank Glorius 1
Affiliation
|
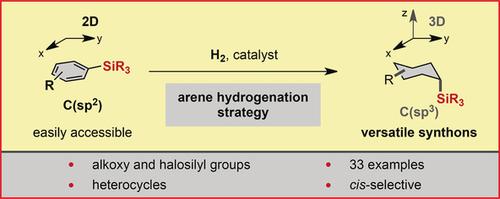
We report a method to convert readily available silylated arenes into silylated saturated carbo‐ and heterocycles by arene hydrogenation. The scope includes alkoxy‐ and halosilyl substituents. Silyl groups can be derivatized into a plethora of functionalities and find application in organic synthesis, materials science, and pharmaceutical, agrochemical, and fragrance research. However, silylated saturated (hetero‐ ) cycles are difficult to access with current technologies. The yield of the hydrogenation depends on the amount of the silica gel additive. This silica effect also enables a significant improvement of a previously disclosed method for the hydrogenation of highly fluorinated arenes (e.g., to all‐cis‐C6H6F6).
中文翻译:

甲硅烷基氢化:一种战略方法,可以直接使用通用的甲硅烷基饱和饱和碳和杂环化合物
我们报告了一种通过芳烃加氢将易得的甲硅烷基化芳烃转化为甲硅烷基化饱和碳和杂环的方法。范围包括烷氧基和卤代甲硅烷基取代基。甲硅烷基可被衍生为多种功能,并在有机合成,材料科学以及制药,农业化学和香料研究中得到应用。但是,目前的技术很难获得甲硅烷基化的饱和(杂)循环。氢化的产率取决于硅胶添加剂的量。这种二氧化硅效应还可以显着改善以前公开的高度氟化芳烃的氢化方法(例如,氢化至全顺式C 6 H 6 F 6)。
更新日期:2018-06-21
中文翻译:

甲硅烷基氢化:一种战略方法,可以直接使用通用的甲硅烷基饱和饱和碳和杂环化合物
我们报告了一种通过芳烃加氢将易得的甲硅烷基化芳烃转化为甲硅烷基化饱和碳和杂环的方法。范围包括烷氧基和卤代甲硅烷基取代基。甲硅烷基可被衍生为多种功能,并在有机合成,材料科学以及制药,农业化学和香料研究中得到应用。但是,目前的技术很难获得甲硅烷基化的饱和(杂)循环。氢化的产率取决于硅胶添加剂的量。这种二氧化硅效应还可以显着改善以前公开的高度氟化芳烃的氢化方法(例如,氢化至全顺式C 6 H 6 F 6)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号