当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Pressure Phase Relations and Crystal Structures of Postspinel Phases in MgV2O4, FeV2O4, and MnCr2O4: Crystal Chemistry of AB2O4 Postspinel Compounds
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00810 Takayuki Ishii 1 , Tsubasa Sakai 1 , Hiroshi Kojitani 1 , Daisuke Mori 1 , Yoshiyuki Inaguma 1 , Yoshitaka Matsushita 2 , Kazunari Yamaura 2 , Masaki Akaogi 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00810 Takayuki Ishii 1 , Tsubasa Sakai 1 , Hiroshi Kojitani 1 , Daisuke Mori 1 , Yoshiyuki Inaguma 1 , Yoshitaka Matsushita 2 , Kazunari Yamaura 2 , Masaki Akaogi 1
Affiliation
|
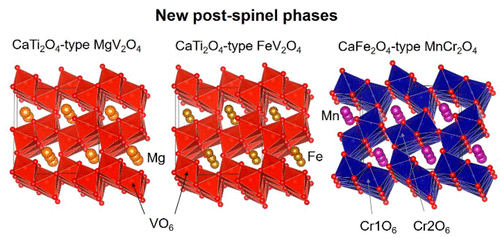
We have investigated high-pressure, high-temperature phase transitions of spinel (Sp)-type MgV2O4, FeV2O4, and MnCr2O4. At 1200–1800 °C, MgV2O4 Sp decomposes at 4–7 GPa into a phase assemblage of MgO periclase + corundum (Cor)-type V2O3, and they react at 10–15 GPa to form a phase with a calcium titanite (CT)-type structure. FeV2O4 Sp transforms to CT-type FeV2O4 at 12 GPa via decomposition phases of FeO wüstite + Cor-type V2O3. MnCr2O4 Sp directly transforms to the calcium ferrite (CF)-structured phase at 10 GPa and 1000–1400 °C. Rietveld refinements of CT-type MgV2O4 and FeV2O4 and CF-type MnCr2O4 confirm that both the CT- and CF-type structures have frameworks formed by double chains of edge-shared B3+O6 octahedra (B3+ = V3+ and Cr3+) running parallel to one of orthorhombic cell axes. A relatively large A2+ cation (A2+ = Mg2+, Fe2+, and Mn2+) occupies a tunnel-shaped space formed by corner-sharing of four double chains. Effective coordination numbers calculated from eight neighboring oxygen–A2+ cation distances of CT-type MgV2O4 and FeV2O4 and CF-type MnCr2O4 are 5.50, 5.16, and 7.52, respectively. This implies that the CT- and CF-type structures practically have trigonal prism (six-coordinated) and bicapped trigonal prism (eight-coordinated) sites for the A2+ cations, respectively. A relationship between cation sizes of VIIIA2+ and VIB3+ and crystal structures (CF- and CT-types) of A2+B23+O4 is discussed using the above new data and available previous data of the postspinel phases. We found that CF-type A2+B23+O4 crystallize in wide ionic radius ranges of 0.9–1.4 Å for VIIIA2+ and 0.55–1.1 Å for VIB3+, whereas CT-type phases crystallize in very narrow ionic radius ranges of ∼0.9 Å for VIIIA2+ and 0.6–0.65 Å for VIB3+. This would be attributed to the fact that the tunnel space of CT-type structure is geometrically less flexible due to the smaller coordination number for A2+ cation than that of CF-type.
中文翻译:

MgV 2 O 4,FeV 2 O 4和MnCr 2 O 4的高压后相关系和后尖晶石相的晶体结构:AB 2 O 4后尖晶石化合物的晶体化学
我们研究了尖晶石(Sp)型MgV 2 O 4,FeV 2 O 4和MnCr 2 O 4的高压高温相变。在1200–1800°C下,MgV 2 O 4 Sp在4–7 GPa处分解为MgO周长石+刚玉(Cor)型V 2 O 3的相组装,它们在10–15 GPa下反应形成与钛酸钙(CT)型结构。FeV 2 O 4 Sp通过FeO辉石+ Cor型V 2 O 3的分解相转变为12 GPa的CT型FeV 2 O 4。。MnCr 2 O 4 Sp在10 GPa和1000–1400°C下直接转变为铁酸钙(CF)结构相。CT型MgV 2 O 4和FeV 2 O 4以及CF型MnCr 2 O 4的Rietveld改进证实,CT型和CF型结构均具有由边缘共享的B 3+ O 6八面体的双链形成的骨架(B 3+= V 3+和Cr 3+)平行于正交晶胞轴之一延伸。相对较大的A 2+阳离子(A 2+ = Mg 2 +,Fe 2+(Mn 2+)占据由四个双链的角共享形成的隧道形空间。根据CT型MgV 2 O 4和FeV 2 O 4和CF型MnCr 2 O 4的八个相邻的氧-A 2+阳离子距离计算的有效配位数分别为5.50、5.16和7.52。这意味着CT和CF型结构实际上分别具有用于A 2+阳离子的三角棱柱(六配位)和三角形三角棱柱(八配位)位。VIII A 2+和VI B 3+的阳离子大小之间的关系利用上述新数据和后松晶石相的现有数据,讨论了A 2+ B 2 3+ O 4的晶体结构(CF型和CT型)。我们发现CF型A 2+ B 2 3+ O 4在VIII A 2+的宽离子半径范围内结晶为0.9–1.4Å,对于VI B 3+在0.55–1.1Å的宽离子半径范围内结晶,而CT型相在非常高的离子半径范围内结晶。VIII A 2+的离子半径范围很窄,约为0.9Å,VI B 3+的离子半径范围很窄,为0.6–0.65Å。这归因于以下事实:由于A 2+阳离子的配位数比CF型小,因此CT型结构的隧道空间在几何上较不灵活。
更新日期:2018-05-23
中文翻译:

MgV 2 O 4,FeV 2 O 4和MnCr 2 O 4的高压后相关系和后尖晶石相的晶体结构:AB 2 O 4后尖晶石化合物的晶体化学
我们研究了尖晶石(Sp)型MgV 2 O 4,FeV 2 O 4和MnCr 2 O 4的高压高温相变。在1200–1800°C下,MgV 2 O 4 Sp在4–7 GPa处分解为MgO周长石+刚玉(Cor)型V 2 O 3的相组装,它们在10–15 GPa下反应形成与钛酸钙(CT)型结构。FeV 2 O 4 Sp通过FeO辉石+ Cor型V 2 O 3的分解相转变为12 GPa的CT型FeV 2 O 4。。MnCr 2 O 4 Sp在10 GPa和1000–1400°C下直接转变为铁酸钙(CF)结构相。CT型MgV 2 O 4和FeV 2 O 4以及CF型MnCr 2 O 4的Rietveld改进证实,CT型和CF型结构均具有由边缘共享的B 3+ O 6八面体的双链形成的骨架(B 3+= V 3+和Cr 3+)平行于正交晶胞轴之一延伸。相对较大的A 2+阳离子(A 2+ = Mg 2 +,Fe 2+(Mn 2+)占据由四个双链的角共享形成的隧道形空间。根据CT型MgV 2 O 4和FeV 2 O 4和CF型MnCr 2 O 4的八个相邻的氧-A 2+阳离子距离计算的有效配位数分别为5.50、5.16和7.52。这意味着CT和CF型结构实际上分别具有用于A 2+阳离子的三角棱柱(六配位)和三角形三角棱柱(八配位)位。VIII A 2+和VI B 3+的阳离子大小之间的关系利用上述新数据和后松晶石相的现有数据,讨论了A 2+ B 2 3+ O 4的晶体结构(CF型和CT型)。我们发现CF型A 2+ B 2 3+ O 4在VIII A 2+的宽离子半径范围内结晶为0.9–1.4Å,对于VI B 3+在0.55–1.1Å的宽离子半径范围内结晶,而CT型相在非常高的离子半径范围内结晶。VIII A 2+的离子半径范围很窄,约为0.9Å,VI B 3+的离子半径范围很窄,为0.6–0.65Å。这归因于以下事实:由于A 2+阳离子的配位数比CF型小,因此CT型结构的隧道空间在几何上较不灵活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号