当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Diimines as Versatile, Derivatizable Ligands in Ruthenium(II) p-Cymene Anticancer Complexes
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00882 Lorenzo Biancalana 1 , Lucinda K. Batchelor 2 , Tiziana Funaioli 1 , Stefano Zacchini 3 , Marco Bortoluzzi 4 , Guido Pampaloni 1 , Paul J. Dyson 2 , Fabio Marchetti 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-23 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00882 Lorenzo Biancalana 1 , Lucinda K. Batchelor 2 , Tiziana Funaioli 1 , Stefano Zacchini 3 , Marco Bortoluzzi 4 , Guido Pampaloni 1 , Paul J. Dyson 2 , Fabio Marchetti 1
Affiliation
|
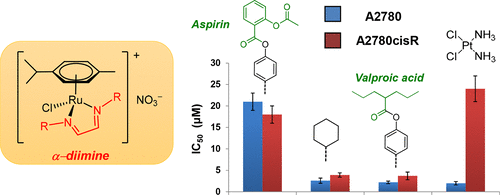
α-Diimines are among the most robust and versatile ligands available in synthetic coordination chemistry, possessing finely tunable steric and electronic properties. A series of novel cationic ruthenium(II) p-cymene complexes bearing simple α-diimine ligands, [(η6-p-cymene)RuCl{κ2N-(HCNR)2}]NO3 (R = Cy, [1]NO3; R = 4-C6H10OH, [2]NO3; R = 4-C6H4OH, [3]NO3), were prepared in near-quantitative yields as their nitrate salts. [2]NO3 displays high water solubility. The potential of the α-diimine ligand in [3]NO3 as a carrier of bioactive molecules was investigated via esterification reactions with the hydroxyl groups. Thus, the double-functionalized derivatives [(η6-p-cymene)RuCl{κ2N-(HCN(4-C6H4OCO-R))2}]NO3 (R = aspirinate, [5]NO3; valproate, [6]NO3) and also [4]Cl (R = Me) were obtained in good-to-high yields. UV–vis and multinuclear NMR spectroscopy and cyclic voltammetric studies in aqueous solution revealed only minor ruthenium chloride hydrolytic cleavage, biologically accessible reduction potentials, and pH-dependent behavior of [3]NO3. Density functional theory analysis was performed in order to compare the Ru–Cl bond strength in [1]+ with the analogous ethylenediamine complex, showing that the higher stability observed in the former is related to the electron-withdrawing properties of the α-diimine ligand. In vitro cytotoxicity studies were performed against tumorigenic (A2780 and A2780cisR) and nontumorigenic (HEK-293) cell lines, with the complexes bearing simple α-diimine ligands ranging from inactive to IC50 values in the low micromolar range. The complexes functionalized with bioactive components, i.e., [5]NO3 and [6]NO3, exhibited a marked increase in the cytotoxicity with respect to the precursor [3]NO3.
中文翻译:

α-二亚胺作为钌(II)-对伞花烃抗癌复合物中的多功能可衍生配体
α-二亚胺是合成配位化学中可用的最强大和最通用的配体之一,具有可微调的空间和电子特性。一系列新的阳离子钌(II)p -cymene络合物轴承简单α二亚胺配体,[(η 6 - p -cymene)的RuCl {κ 2 Ñ - (HCNR)2 }] NO 3(R = CY,[ 1 ] NO 3; R = 4-C 6 H 10 OH,[ 2 ] NO 3; R = 4-C 6 H 4 OH,[ 3 ] NO 3)以接近定量的形式制备为它们的硝酸盐。[ 2 ] NO 3显示出高水溶性。通过与羟基的酯化反应,研究了[ 3 ] NO 3中α-二亚胺配体作为生物活性分子载体的潜力。因此,双官能化的衍生物[(η 6 - p -cymene)的RuCl {κ 2 Ñ - (HCN(4-C 6 H ^ 4 OCO-R))2 }] NO 3(R =阿司匹林,[ 5 ] NO 3 ;丙戊酸盐,[ 6 ] NO 3),还有[ 4以高到高的产率获得] Cl(R = Me)。水溶液中的紫外可见光谱和多核NMR光谱法以及循环伏安法研究表明,氯化钌仅能进行少量水解裂解,生物上可还原的还原电位以及[ 3 ] NO 3的pH依赖行为。为了比较[ 1 ] +中的Ru-Cl键强度,进行了密度泛函理论分析。与类似的乙二胺配合物相比,表明在前者中观察到的更高的稳定性与α-二亚胺配体的吸电子性能有关。进行了针对致瘤性(A2780和A2780cisR)和非致瘤性(HEK-293)细胞系的体外细胞毒性研究,这些复合物带有简单的α-二亚胺配体,范围从无活性到低微摩尔范围内的IC 50值。用生物活性成分即[ 5 ] NO 3和[ 6 ] NO 3功能化的复合物相对于前体[ 3 ] NO 3表现出明显的细胞毒性。
更新日期:2018-05-23
中文翻译:

α-二亚胺作为钌(II)-对伞花烃抗癌复合物中的多功能可衍生配体
α-二亚胺是合成配位化学中可用的最强大和最通用的配体之一,具有可微调的空间和电子特性。一系列新的阳离子钌(II)p -cymene络合物轴承简单α二亚胺配体,[(η 6 - p -cymene)的RuCl {κ 2 Ñ - (HCNR)2 }] NO 3(R = CY,[ 1 ] NO 3; R = 4-C 6 H 10 OH,[ 2 ] NO 3; R = 4-C 6 H 4 OH,[ 3 ] NO 3)以接近定量的形式制备为它们的硝酸盐。[ 2 ] NO 3显示出高水溶性。通过与羟基的酯化反应,研究了[ 3 ] NO 3中α-二亚胺配体作为生物活性分子载体的潜力。因此,双官能化的衍生物[(η 6 - p -cymene)的RuCl {κ 2 Ñ - (HCN(4-C 6 H ^ 4 OCO-R))2 }] NO 3(R =阿司匹林,[ 5 ] NO 3 ;丙戊酸盐,[ 6 ] NO 3),还有[ 4以高到高的产率获得] Cl(R = Me)。水溶液中的紫外可见光谱和多核NMR光谱法以及循环伏安法研究表明,氯化钌仅能进行少量水解裂解,生物上可还原的还原电位以及[ 3 ] NO 3的pH依赖行为。为了比较[ 1 ] +中的Ru-Cl键强度,进行了密度泛函理论分析。与类似的乙二胺配合物相比,表明在前者中观察到的更高的稳定性与α-二亚胺配体的吸电子性能有关。进行了针对致瘤性(A2780和A2780cisR)和非致瘤性(HEK-293)细胞系的体外细胞毒性研究,这些复合物带有简单的α-二亚胺配体,范围从无活性到低微摩尔范围内的IC 50值。用生物活性成分即[ 5 ] NO 3和[ 6 ] NO 3功能化的复合物相对于前体[ 3 ] NO 3表现出明显的细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号