当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
RATIONALIZING THE REGIOSELECTIVITY OF CATIONIC RING-OPENING POLYMERIZATION OF BENZOXAZINES
European Polymer Journal ( IF 5.8 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.eurpolymj.2018.05.024 Tuğba Furuncuoğlu Özaltın , Saron Catak , Baris Kiskan , Yusuf Yagci , Viktorya Aviyente
European Polymer Journal ( IF 5.8 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.eurpolymj.2018.05.024 Tuğba Furuncuoğlu Özaltın , Saron Catak , Baris Kiskan , Yusuf Yagci , Viktorya Aviyente
|
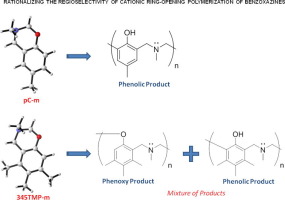
Abstract Polybenzoxazines have gained increasing interest both in industry and academia for the last few decades due to their unique structural features. The ring-opening polymerization mechanism of 1,3-benzoxazine monomers and the regioselectivity during polymerization, still need further clarification. In this study, ring-opening polymerization mechanisms of two methyl substituted benzoxazine derivatives 3,6-dimethyl-3,4-dihydro-2H-benzo[e][1,3] oxazine (pC-m) and 3,5,6,7-tetramethyl-3,4-dihydro-2Hbenzo[e][1,3] oxazine (345TMP-m) are investigated by quantum mechanical tools using density functional theory (DFT). Calculations have shown that in the presence of a nucleophile, pC-m can yield the phenolic polymer upon rearrangement of its intermediate phenoxy product. However, the polymerization of 345TMP-m results in a mixture of phenoxy and phenolic type polymers. The extra methyl groups on 345TMP-m have a dual role in preventing the π stacking interactions observed in pC-m, and in decreasing the barrier yielding phenolic polymers by electron donation.
中文翻译:

合理化苯并恶嗪阳离子开环聚合的区域选择性
摘要 在过去的几十年里,聚苯并恶嗪因其独特的结构特征而在工业界和学术界受到越来越多的关注。1,3-苯并恶嗪单体的开环聚合机理和聚合过程中的区域选择性,仍有待进一步阐明。在本研究中,两种甲基取代的苯并恶嗪衍生物 3,6-二甲基-3,4-二氢-2H-苯并[e][1,3] 恶嗪 (pC-m) 和 3,5,6 的开环聚合机理,7-四甲基-3,4-二氢-2Hbenzo[e][1,3] oxazine (345TMP-m) 通过量子力学工具使用密度泛函理论 (DFT) 进行研究。计算表明,在亲核试剂的存在下,pC-m 可以在其中间体苯氧基产物重排后产生酚类聚合物。然而,345TMP-m 聚合生成苯氧基和酚类聚合物的混合物。345TMP-m 上的额外甲基具有双重作用,可防止在 pC-m 中观察到的 π 堆积相互作用,以及通过给电子降低产生酚类聚合物的势垒。
更新日期:2018-08-01
中文翻译:

合理化苯并恶嗪阳离子开环聚合的区域选择性
摘要 在过去的几十年里,聚苯并恶嗪因其独特的结构特征而在工业界和学术界受到越来越多的关注。1,3-苯并恶嗪单体的开环聚合机理和聚合过程中的区域选择性,仍有待进一步阐明。在本研究中,两种甲基取代的苯并恶嗪衍生物 3,6-二甲基-3,4-二氢-2H-苯并[e][1,3] 恶嗪 (pC-m) 和 3,5,6 的开环聚合机理,7-四甲基-3,4-二氢-2Hbenzo[e][1,3] oxazine (345TMP-m) 通过量子力学工具使用密度泛函理论 (DFT) 进行研究。计算表明,在亲核试剂的存在下,pC-m 可以在其中间体苯氧基产物重排后产生酚类聚合物。然而,345TMP-m 聚合生成苯氧基和酚类聚合物的混合物。345TMP-m 上的额外甲基具有双重作用,可防止在 pC-m 中观察到的 π 堆积相互作用,以及通过给电子降低产生酚类聚合物的势垒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号