当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of highly enantiopure β,β-diaryl substituted glycine containing two contiguous stereocenters via asymmetric 1,6-conjugate addition
Tetrahedron ( IF 2.1 ) Pub Date : 2018-05-22 , DOI: 10.1016/j.tet.2018.05.057 Junhua Tong , Liang Zhao , Huihui Li , Chenglin Wu , Xu Han , Jiang Wang , Hong Liu
中文翻译:

通过不对称的1,6-共轭加成构建具有两个连续立体中心的高度对映体纯的β,β-二芳基取代的甘氨酸
更新日期:2018-05-22
Tetrahedron ( IF 2.1 ) Pub Date : 2018-05-22 , DOI: 10.1016/j.tet.2018.05.057 Junhua Tong , Liang Zhao , Huihui Li , Chenglin Wu , Xu Han , Jiang Wang , Hong Liu
|
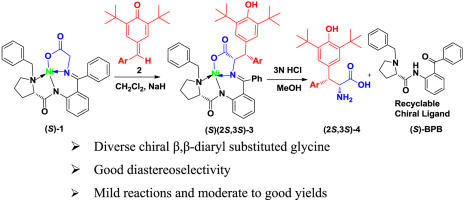
Asymmetric construction of β,β-diaryl substituted glycine bearing two contiguous chiral centers remains a significant challenge. Herein, the application of the para-quinone methides (p-QMs) and Ni(II)- complex of glycine via 1,6-conjugate addition to achieve asymmetric synthesis of β,β-diaryl substituted glycine has been explored successfully. This protocol features operational simplicity, high diastereoselectivity, and recyclable chiral ligand, which provides a versatile approach to the synthesis of highly enantiopure β,β-diaryl substituted glycine.
中文翻译:

通过不对称的1,6-共轭加成构建具有两个连续立体中心的高度对映体纯的β,β-二芳基取代的甘氨酸
带有两个连续手性中心的β,β-二芳基取代的甘氨酸的不对称结构仍然是一个重大挑战。在本文中,已经成功地探索了通过1,6-共轭加成反应对甘氨酸的对醌甲基化物(p -QMs)和Ni(II)-络合物的应用,以实现β,β-二芳基取代的甘氨酸的不对称合成。该方案具有操作简便,高非对映选择性和可回收的手性配体的特点,为合成高对映体纯的β,β-二芳基取代的甘氨酸提供了一种通用方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号