当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thorpe–Ingold Effect in Branch‐Selective Alkylation of Unactivated Aryl Fluorides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804479 Matthew J. O'Neill 1 , Tim Riesebeck 1 , Josep Cornella 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804479 Matthew J. O'Neill 1 , Tim Riesebeck 1 , Josep Cornella 1
Affiliation
|
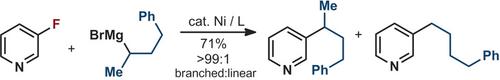
Presented herein is a general protocol for the alkylation of simple aryl fluorides with unbiased secondary Grignard reagents by means of nickel catalysis. This study revealed a general Thorpe–Ingold effect in the ligand backbone which confers a high degree of selectivity for the secondary carbon center in the C−C coupling event. This protocol is characterized by mild reaction conditions, robustness, and simplicity. Both electron‐rich and electron‐deficient aryl fluorides are suitable candidates in this transformation. Equally amenable are a variety of heterocycles, permitting the coupling without over alkylation at the electrophilic sites.
中文翻译:

索普-英戈尔德效应在未活化芳基氟化物的支链选择性烷基化中
本文提出了通过镍催化用无偏的二次格氏试剂将简单的芳基氟化物烷基化的一般方案。这项研究揭示了配体主链上普遍的索普-英戈尔德效应,在CC偶合事件中赋予了次级碳中心高度的选择性。该协议的特点是反应条件温和,稳健且简单。富电子和缺电子的芳基氟化物都是该转化的合适候选物。同样适合的是各种杂环,允许偶联而在亲电子位点没有过度烷基化。
更新日期:2018-06-19
中文翻译:

索普-英戈尔德效应在未活化芳基氟化物的支链选择性烷基化中
本文提出了通过镍催化用无偏的二次格氏试剂将简单的芳基氟化物烷基化的一般方案。这项研究揭示了配体主链上普遍的索普-英戈尔德效应,在CC偶合事件中赋予了次级碳中心高度的选择性。该协议的特点是反应条件温和,稳健且简单。富电子和缺电子的芳基氟化物都是该转化的合适候选物。同样适合的是各种杂环,允许偶联而在亲电子位点没有过度烷基化。










































 京公网安备 11010802027423号
京公网安备 11010802027423号