当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Conversion of Planar trans-Chlorins into Highly Twisted Doubly Fused Porphyrins or Chlorins via Oxidative Fusion
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00849 Nivedita Chaudhri 1 , Nitika Grover 1 , Muniappan Sankar 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00849 Nivedita Chaudhri 1 , Nitika Grover 1 , Muniappan Sankar 1
Affiliation
|
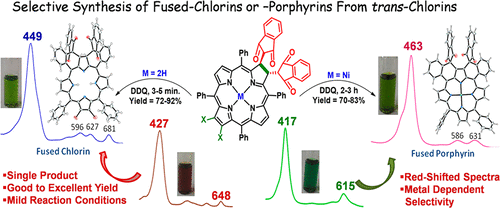
β-to-o-phenyl doubly fused porphyrins (DFPs) or chlorins (DFCs) were selectively synthesized by facile oxidative fusion of trans-chlorins using 2,3-dichloro-5,6-dicyano-1,4- benzoquinone (DDQ) in good-to-excellent yields (70–92%) under mild reaction conditions with high atom economy. The selectivity in product formation (difused porphyrin or chlorin) was controlled by the presence or absence of a Ni(II) ion in the macrocyclic core. Notably, nickel(II) trans-chlorins selectively yielded DFPs, whereas free-base trans-chlorins afforded only DFCs. The synthesized fused porphyrinoids exhibited significantly red-shifted electronic spectral features (Δλmax = 16–53 nm) of the Soret band due to the extended π conjugation and highly twisted macrocyclic conformation (twist angle ∼20–34°). Inner-core NHs of fused chlorins exhibited a tremendous downfield shift (Δδ = 1.71–2.02 ppm) compared to their precursors. The overall protonation constants for indanedione-substituted free-base-difused chlorins (4–6) were profoundly higher (∼20–50-fold) compared to dicyanomethyl-appended free-base-difused chlorins (10–12) because of the combined effect of the electronic nature of the β-substituents and nonplanarity of the macrocyclic core. The first oxidation potential of H2DFC(MN)2Ph2 (12) was 0.54 V cathodically shifted with respect to H2DFC(MN)2 (10) because of the electron-donating nature of the β-phenyl groups, which resulted in extensive destabilization of the highest occupied molecular orbital.
中文翻译:

通过氧化融合将平面反式氯霉素选择性转化为高度扭曲的双融合卟啉或氯霉素
通过使用2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)轻松进行反式二氢卟酚的氧化融合,选择性地合成了β-邻-苯基双稠合卟啉(DFP)或二氢卟酚(DFC )。在温和的反应条件下以高原子经济性获得了良好至优异的产率(70-92%)。通过大环核中是否存在Ni(II)离子来控制产物形成的选择性(稀卟啉或二氢卟酚)。值得注意的是,镍(II)反式-二氢卟酚选择性地产生DFP,而游离碱反式-二氢卟酚仅提供DFC。合成的稠合卟啉类化合物显示出明显的红移电子光谱特征(最大Δλ由于扩展了π共轭和高度扭曲的大环构象(扭转角约为20-34°),所以Soret谱带为= 16–53 nm)。与它们的前驱体相比,稠合二氢卟酚的核心NHs表现出极大的低场偏移(Δδ= 1.71–2.02 ppm)。茚二酮取代的游离碱扩散的二氢卟酚的整体质子化常数(4 – 6)比双氰基甲基游离碱扩散的二氢卟酚(10 – 12)高得多(约20–50倍)。β取代基的电子性质和大环核的非平面性的影响。H 2 DFC(MN)2 Ph 2(12)由于β-苯基的供电子性而相对于H 2 DFC(MN)2(10)阴极移动了0.54 V ,这导致最高占据分子轨道的广泛失稳。
更新日期:2018-05-22
中文翻译:

通过氧化融合将平面反式氯霉素选择性转化为高度扭曲的双融合卟啉或氯霉素
通过使用2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)轻松进行反式二氢卟酚的氧化融合,选择性地合成了β-邻-苯基双稠合卟啉(DFP)或二氢卟酚(DFC )。在温和的反应条件下以高原子经济性获得了良好至优异的产率(70-92%)。通过大环核中是否存在Ni(II)离子来控制产物形成的选择性(稀卟啉或二氢卟酚)。值得注意的是,镍(II)反式-二氢卟酚选择性地产生DFP,而游离碱反式-二氢卟酚仅提供DFC。合成的稠合卟啉类化合物显示出明显的红移电子光谱特征(最大Δλ由于扩展了π共轭和高度扭曲的大环构象(扭转角约为20-34°),所以Soret谱带为= 16–53 nm)。与它们的前驱体相比,稠合二氢卟酚的核心NHs表现出极大的低场偏移(Δδ= 1.71–2.02 ppm)。茚二酮取代的游离碱扩散的二氢卟酚的整体质子化常数(4 – 6)比双氰基甲基游离碱扩散的二氢卟酚(10 – 12)高得多(约20–50倍)。β取代基的电子性质和大环核的非平面性的影响。H 2 DFC(MN)2 Ph 2(12)由于β-苯基的供电子性而相对于H 2 DFC(MN)2(10)阴极移动了0.54 V ,这导致最高占据分子轨道的广泛失稳。











































 京公网安备 11010802027423号
京公网安备 11010802027423号