当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Aryl Nitriles and 3-Imino-1-oxoisoindolines via Nickel-Promoted C(sp2)–H Cyanations
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1021/acs.orglett.8b01056 Lin Yu 1 , Xiang Chen 1 , Ze-Nan Song 1 , Da Liu 1 , Liang Hu 1 , Yongqi Yu 1 , Ze Tan 1 , Qingwen Gui 2
Organic Letters ( IF 5.2 ) Pub Date : 2018-05-22 00:00:00 , DOI: 10.1021/acs.orglett.8b01056 Lin Yu 1 , Xiang Chen 1 , Ze-Nan Song 1 , Da Liu 1 , Liang Hu 1 , Yongqi Yu 1 , Ze Tan 1 , Qingwen Gui 2
Affiliation
|
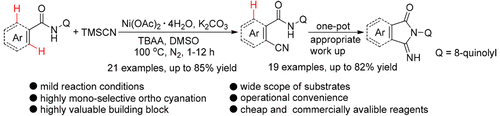
An efficient nickel-promoted selective monocyanation of benzamides with TMSCN via 8-aminoquinoline directed ortho C–H activation has been developed. Varieties of functionalized ortho-cyanated (hetero)aryl nitriles can be selectively synthesized in moderate to good yields. These cyanation products can be easily transformed into various 3-imino-1-oxoisoindolines in a one-pot procedure. The mild reaction conditions, use of cheap and commercially available reagents, wide functional group tolerance, and operational convenience make this protocol practical to the synthetic community.
中文翻译:

镍促进的C(sp 2)–H氰基选择性合成芳基腈和3-Imino-1-氧代异吲哚啉
已经开发出一种有效的镍促进的苯甲酰胺通过8-氨基喹啉直接邻位C–H活化与TMSCN选择性单氰化的方法。可以以中等至良好的产率选择性地合成各种官能化的正氰基化(杂)芳基腈。这些氰化产物可以通过一锅法轻松地转化为各种3-亚氨基-1-氧代异吲哚啉。温和的反应条件,使用便宜的和可商购的试剂,宽泛的官能团耐受性和操作方便性使该方案对合成界可行。
更新日期:2018-05-22
中文翻译:

镍促进的C(sp 2)–H氰基选择性合成芳基腈和3-Imino-1-氧代异吲哚啉
已经开发出一种有效的镍促进的苯甲酰胺通过8-氨基喹啉直接邻位C–H活化与TMSCN选择性单氰化的方法。可以以中等至良好的产率选择性地合成各种官能化的正氰基化(杂)芳基腈。这些氰化产物可以通过一锅法轻松地转化为各种3-亚氨基-1-氧代异吲哚啉。温和的反应条件,使用便宜的和可商购的试剂,宽泛的官能团耐受性和操作方便性使该方案对合成界可行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号